Cambridge Healthtech Institute’s 3rd
Biospecimen, Central Lab and Technology for Precision Medicine Trials:
Infrastructure to Support Biomarker-Driven Clinical Research
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
The availability of high quality biological specimens, laboratory access and diagnostics services are of utmost importance for biomarker-driven clinical trials and future research. The complexity and number of samples collected during studies has increased
steadily over the years and we need to come up with best practices, operational models and IT systems to deal with this volume and complexity. The next step, the testing of the samples and various laboratory services also require significant managerial
efforts whether they are outsourced or provided by an in-house laboratory. Cambridge Healthtech Institute’s 3rd Annual Biospecimen, Central Lab and Technology for Precision Medicine Trials conference brings together leading
experts to discuss challenges and identify actions to improve infrastructure for biomarker driven clinical trials.
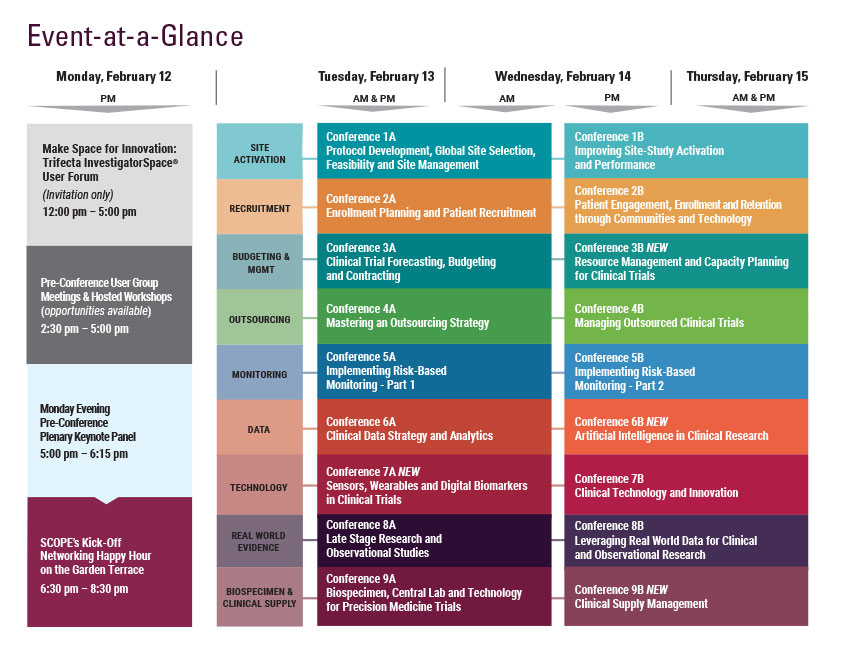
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Michael Tanen, Director, Clinical Biomarker Specimen Management, Merck
10:50 Precision Medicine Trials, and How the Bio-Repository Can Support Biomarker and Diagnostic Development
 Michael Tanen, Director, Clinical Biomarker Specimen Management, Merck
Michael Tanen, Director, Clinical Biomarker Specimen Management, Merck
Personalized medicine initiatives have led to a marked increase in biomarker-driven research objectives within clinical trials. This has re-defined traditional biospecimen management into a more comprehensive information management role requiring
innovative technology and best practices. The ability to integrate disparate data sources into centralized systems and present the information in a way that can guide decision-making and biomarker development, will define the utility and
success of the biorepository.
11:15 Clinical Biomarker Sample Management: Taking Time to Do It Right, Rather than Do It Over
 Dmitri Mikhailov, Ph.D., Director, Head, Biomarker Study Coordination, Novartis
Dmitri Mikhailov, Ph.D., Director, Head, Biomarker Study Coordination, Novartis
The emergence of global clinical trials with complex biomarker analyses performed at CROs pushes the industry to reconsider clinical sample management. How to mitigate risks of losing samples or compromising quality? How to maximize sample
usage beyond clinical study objectives? This presentation discusses how Novartis approaches these challenges, starting with trial documentation setup, to systems used in biorepository for sample inventory and enabling additional research
use of clinical samples.
11:40 Operationalizing Precision Medicine Trials in Oncology
 Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist,
Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research Laboratories
Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist,
Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research Laboratories
The immuno-oncology revolution has required many sponsors to rethink the infrastructure needed to support complex biomarker driven trials. This presentation will discuss challenges and solutions in operationalizing such trials, including governance
committees, roles and responsibilities of functional areas, biomarker plan structures, and vendor alignment.
12:05 pm Q & A with Speakers
12:30 Session Break
 12:40 Luncheon Presentation: Best Practices in Clinical Trial Samples & Consent Tracking
12:40 Luncheon Presentation: Best Practices in Clinical Trial Samples & Consent Tracking
 Jian Wang, Ph.D., CEO, BioFortis, Inc.
Jian Wang, Ph.D., CEO, BioFortis, Inc.
In biomarker-driven precision medicine clinical trials, patient samples (biospecimens) are as important as patients themselves. Sample assay results often determine patient segmentation, and support primary and key secondary objectives. The
lack of proper sample tracking & management escalate risks of milestone delays and regulatory scrutiny. We illustrate best practices and technology solutions that address the rise in complexity and importance of biospecimen operations,
with unique perspectives from risk-based monitoring approaches.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Jonathan Reuter, Associate Director Global Procurement R&D, Clinical Labs, Bristol-Myers Squibb
2:05 Use of Make vs. Buy Analyses and “Should-Cost” Modeling in Clinical Laboratory Procurement
Jonathan Reuter, Associate Director, Global Procurement R&D, Clinical Labs, Bristol Meyers-Squibb
Elevating the role of procurement in company decision-making. Using comprehensive analysis to enable informed sourcing decisions. Partnering with external supply base to implement cost-controlled and sustainable models in support of the clinical
development cycle.
2:30 Comprehensive Bio-Inventory System to Support Clinical Biomarker Strategy
 Sandra Hudgens, MT(ASCP), BS, MBA, Associate Principal Scientist, Clinical Specimen Management Operations,
Merck
Sandra Hudgens, MT(ASCP), BS, MBA, Associate Principal Scientist, Clinical Specimen Management Operations,
Merck
Precision medicine is a key component to drug development strategy. The management of clinical specimens to support genetic and biomarker testing is critical to realizing precision medicine. This includes having a biospecimen inventory management
system which can seamlessly link specimens to informed consent and clinical data to appropriately select specimens for analysis. Based on established business criteria, the biospecimen inventory management system should be able to track
the specimen life cycle to provide a cost-effective inventory for the organization.
 2:55
Frozen Clinical Biospecimens – New Approach for Optimizing Quality, Compliance, and Cost
2:55
Frozen Clinical Biospecimens – New Approach for Optimizing Quality, Compliance, and Cost
 Robert Sever, PhD, Associate Director, Research & Development, Praxair, Inc.
Robert Sever, PhD, Associate Director, Research & Development, Praxair, Inc.
This seminar will review the challenges of today’s cold chain for clinical biospecimens from kit preparation and dry ice management to sample acquisition and shipment logistics. A new approach will be described that can improve sample
preservation, process compliance, and productivity while reducing overall complexity, risk, and cost.
3:10 Sponsored Presentation (Opportunity Available)
3:25 PANEL DISCUSSION: Biospecimen and Core Lab Considerations for Risk-Based Monitoring
Moderator: Michael Tanen, Director, Clinical Biomarker Specimen Management, Merck
Panelists: Jonathan Reuter, Associate Director Global Procurement R&D, Clinical Labs, Bristol-Myers Squibb
Jian Wang, Ph.D., CEO, BioFortis, Inc.
Dmitri Mikhailov, Ph.D., Director, Head, Biomarker Study Coordination, Novartis
Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist, Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research Laboratories
- Importance of Biospecimens in Precision Medicine trials
- When patient samples are as important as the patient themselves
- Specimen-centric RBM approaches
- Inclusion of specimen KRIs (key risk indicators)
- Working with RMB colleagues to improve quality
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest
and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and
problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove such
concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice technology
and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Lynn Wetherwax, Senior Manager, Biobank, Translational Sciences Operations, Amgen
8:30 CO-PRESENTATION: Clinical Sample Tracking Project Implementation: A Case Study
 Ron Bourque, Associate Director, RDI, Clinical Biologics, MedImmune/AstraZeneca
Ron Bourque, Associate Director, RDI, Clinical Biologics, MedImmune/AstraZeneca
Venkatraman Raman, Senior Project Manager, R&D Information, Clinical Biologics, AstraZeneca
We have developed a new and innovative sample management model combining MedImmune Clinical Operations with close alliance/partnership to a Central Lab. Together the technology we are employing is Labmatrix. This initiative is focused
on accepting standardized data from all lab vendors. Discrepant data will be corrected at the source lab and reflected back into the tool. Labmatrix is also receiving consent data from our EDC.
8:55 Enabling Scientific Discovery and Innovation Using Biomarker Specimens by Means of Advanced Informatics
 Lynn Wetherwax, Senior Manager, Biobank, Translational Sciences Operations, Amgen
Lynn Wetherwax, Senior Manager, Biobank, Translational Sciences Operations, Amgen
Biomarker specimens may be collected with a specific purpose in mind or they may be stored until that “a-ha” moment when scientific discovery hinges on biomarker investigation. This presentation will provide an overview of
biomarker specimen management strategy using informatics to track, confirm consent and search clinical data attributes related to available specimens.
9:20 Leveraging Systems and Automation to Address the Challenges in GSK’s Sample Management Strategy Implementation
 Kimberly Bojczuk, Investigator, Discovery Supply - Global Biological Assets, RD Platform Technology &
Science, GSK
Kimberly Bojczuk, Investigator, Discovery Supply - Global Biological Assets, RD Platform Technology &
Science, GSK
GSK is developing a cross-functional strategy working with IT and global teams, to increase the visibility and use of human biomaterials in Discovery and Clinical. This integrated approach will increase standardization, allow GSK to maximize
investment in biological materials and ensure increased compliance. An IT platform to provide a single interface to both on-site and off-site storage, as well as rapid delivery of samples from an automated sample store will facilitate
addressing challenges faced.
 9:45 Bringing the Trial to the Patient: Sample Collection at Home for Clinical Trials
9:45 Bringing the Trial to the Patient: Sample Collection at Home for Clinical Trials
 Kevin Bateman, Distinguished Scientist, Pharmacokinetics, Pharmacodynamics and Drug Metabolism, Merck & Co.
Kevin Bateman, Distinguished Scientist, Pharmacokinetics, Pharmacodynamics and Drug Metabolism, Merck & Co.
Merck's “Smart Trials” initiative evaluates and implements technologies bringing clinical trials to the patient. Enabling this is the ability to collect samples for pharmacokinetic/biomarker analysis away from the clinic. Dried
blood collection is being investigated and learnings will be shared on hurdles met and how they are being addressed.
10:00 Sponsored Presentation (Opportunity Available)
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Brenda Yanak, Global Head, Specimen Strategy & Innovation, End-to-End Specimen Management, Q² Solutions
11:15 Biospecimen Tracking as an Integral Part of Risk Based Monitoring
 Morten Pedersen,, M.Sc. (Pharm), GDBA, Senior Manager, Novo Nordisk
Morten Pedersen,, M.Sc. (Pharm), GDBA, Senior Manager, Novo Nordisk
Novo Nordisk has implemented Risk Based Monitoring with biospecimen tracking being an integral part of the approach. With biospecimens being the primary endpoints in many trials, we took the approach of centrally monitoring the collection
and analysis of these to be able to do a targeted follow up with the sites where results of biospecimen samples were missing. Combining data from several sources, we have set up a system to predict where the risk may emerge and
do a proactive targeted follow-up.
11:40 PANEL DISCUSSION: Outsourcing Strategy: Working with Central Labs and Biorepositories
Moderator:
 Brenda Yanak, Global Head, Specimen Strategy & Innovation, End-to-End Specimen
Management, Q² Solutions
Brenda Yanak, Global Head, Specimen Strategy & Innovation, End-to-End Specimen
Management, Q² Solutions
Panelists:
 Morten Pedersen,, M.Sc. (Pharm), GDBA, Senior Manager, Novo Nordisk
Morten Pedersen,, M.Sc. (Pharm), GDBA, Senior Manager, Novo Nordisk
 Kimberly Bojczuk, Investigator, Discovery Supply - Global Biological Assets, RD Platform Technology
& Science, GSK
Kimberly Bojczuk, Investigator, Discovery Supply - Global Biological Assets, RD Platform Technology
& Science, GSK
 Sandra Hudgens, MT(ASCP), BS, MBA, Associate Principal Scientist, Clinical Specimen Management
Operations, Merck
Sandra Hudgens, MT(ASCP), BS, MBA, Associate Principal Scientist, Clinical Specimen Management
Operations, Merck
- Key Areas of Interest from Sponsors
- What Vendors Wish Sponsors Knew
- Innovation Opportunities
12:05 pm Session Break
12:10 Enjoy Lunch on Your Own
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Marina Filshtinsky, M.D.
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1)
781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com