Cambridge Healthtech Institute’s 7th Annual
Clinical Technology and Innovation:
Disruptive Technologies for Data and Trial Management
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
Digital technology, mobile solutions, novel data collection modalities and integrative systems are becoming game-changing features of modern clinical trials. However, the adoption of novel technology solutions to improve overall outcomes and garner operational
efficiencies has been slower than expected. Cambridge Healthtech Institute’s 7th Annual Clinical Technology and Innovation conference will feature a broad array of topics such as blockchain technology, machine
learning, digital trends, and their adoption and implementation in clinical research. We are looking forward to hosting a practical and productive knowledge and experience exchange.
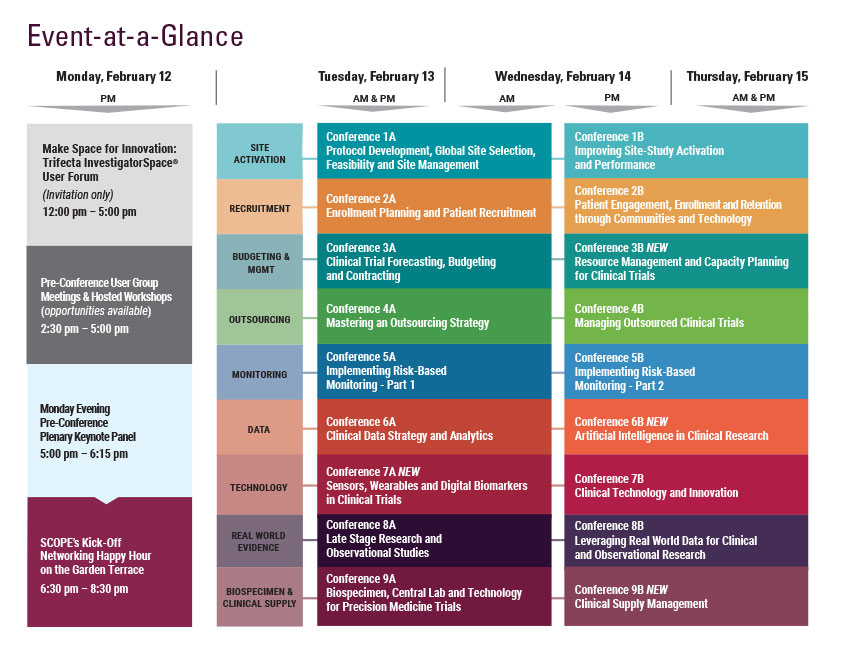
Wednesday, February 14
11:30 am Registration Open
12:10 pm Bridging Luncheon Presentation: Introducing Consent Engineering: The Simplest and Safest Way to Create, Manage and Automate Consent Solutions In-House
 Eric Delente, President, Patient Consent, DrugDev (an IQVIA company)
Eric Delente, President, Patient Consent, DrugDev (an IQVIA company)
The benefits eConsent provides for patient satisfaction and internal efficiencies cannot be overstated, yet the time and expense involved can make it cost-prohibitive for some clinical trials. Consent Engineering is a new SaaS technology solution
enabling sponsors to bring eConsent entirely in-house. Featuring an interface as familiar and intuitive as Microsoft Word, Consent Engineering will completely change the way the industry does consent. Join us for the world’s first look
at this exciting new solution!
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
Balazs Flink, M.D., Clinical Trial Analytics Lead, R&D Business Insights and Analytics, Bristol-Myers Squibb
4:05 Blockchain Disruption: How Blockchain Will Change Our Industry
 Munther Baara, Senior Director, Development Business Technology, Pfizer
Munther Baara, Senior Director, Development Business Technology, Pfizer
Imagine a solution that makes it easy to aggregate health data in a secure, trusted, automated, and error-free way. A solution which enforces rules, privacy, and regulations in a mutually agreed upon manner, resulting in a smart-contract between
patient and healthcare stakeholders. This enables patients to aggregate their data from diverse health sources and share what they choose to with their physicians and researchers. All this puts the patient in control of their health and wellbeing,
rather than being along for the ride: How it works; Key benefits; Empowering the patients with control over their data.
4:30 Exploration of Where Machine Learning Will Help in the Product Development Process in the Pharmaceutical Industry
 Francis Kendall, Technology Evaluation and Implementation Leader, Product Development, Roche
Francis Kendall, Technology Evaluation and Implementation Leader, Product Development, Roche
The talk will explore how Machine Learning is and will change how Product Development is carried out in the industry from improving efficiencies, gaining more insights on products, improved surveillance of products, especially safety and its use
in IoT devices.
4:55 CO-PRESENTATION: Leveraging Digital Transformation to Unify Process, Connect Data, and Turbocharge Innovation in Clinical Operations
 Evi Cohen, Vice President, Global Pharma & Life Sciences, Appian
Evi Cohen, Vice President, Global Pharma & Life Sciences, Appian
 Mike Montello, Vice President, Global Head, R&D Solutions Information Technology, IQVIA
Mike Montello, Vice President, Global Head, R&D Solutions Information Technology, IQVIA
Conducting and managing a successful, safe clinical trial is complicated. With massive data and complex processes at the core, it’s no surprise innovation in Business Process Management (BPM) is behind many successful trials.
5:25 Intelligent Clinical Trial Design, Planning and Conduct
 Balazs Flink, M.D., Clinical Trial Analytics Lead, R&D Business Insights and Analytics, Bristol-Myers
Squibb
Balazs Flink, M.D., Clinical Trial Analytics Lead, R&D Business Insights and Analytics, Bristol-Myers
Squibb
As technology evolves and AI solutions become more sophisticated, there is a natural demand to test and apply them in areas that were traditionally expert opinion-guided. This presentation is about early experiences and challenges of pharma
companies - including BMS - that are starting to apply AI in the R&D space to promote precision oncology, identify targets, design and plan trials and translate strategy to efficient operational execution.
 5:50 Reception Hosted by Cognizant Technology Solutions
5:50 Reception Hosted by Cognizant Technology Solutions
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation,
we will go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
Julie Dietrich, MS, Director, Development Design Center, Amgen
8:35 Remote Data Monitoring: Ideal Model versus Current Practices
 Charlie Romano, Vice President, Global Clinical Operations, Peachtree Bioresearch Solutions
Charlie Romano, Vice President, Global Clinical Operations, Peachtree Bioresearch Solutions
This presentation will discuss qualitative and quantitative research conducted by the Clinical Trials Transformation Initiative to understand the perspectives of patients and site investigators of mobile clinical trials. Discussion will
include insights and advice from site investigators who have participated in trials that incorporate mobile devices to collect data for study endpoints. Key findings from a survey of patients in four therapeutic areas will also be
presented, focusing on factors important to the design and conduct of clinical trials involving mobile devices.
9:00 CO-PRESENTATION: How Technology Can Enable Patient-Centric Clinical Research
 Julie Dietrich, MS, Director, Development Design Center, Amgen
Julie Dietrich, MS, Director, Development Design Center, Amgen
Sindhya Govind, Specialist Business Systems Analyst, Amgen
Presentation highlights will include: Opportunities for technology to be part of a patient-centric strategy and improve patients’ experience with clinical research.
 9:25 Achieving Process Efficiencies & Compliance Throughout the Clinical Supply Chain - Reducing Risk & Streamlining Operations
9:25 Achieving Process Efficiencies & Compliance Throughout the Clinical Supply Chain - Reducing Risk & Streamlining Operations
 Theodora Sarver, Manager, Product Management, Almac Clinical Technologies
Theodora Sarver, Manager, Product Management, Almac Clinical Technologies
Until now, managing accountability and returns has been a tedious, time consuming, and error prone process due to a lack of compliance, standardization, and fragmented record keeping. During this session, we will explore today’s
most holistic solution for accountability and reconciliation tracking. This session will show you how to: - Strengthen and improve site compliance - Cut costs and speed study close-out - Produce and maintain complete and detailed
records across the chain of custody.

 9:50
CO-PRESENTATION: Real World Innovation and Application in Pipeline Data Approaches
9:50
CO-PRESENTATION: Real World Innovation and Application in Pipeline Data Approaches
 Kate Dugan, Vice President, Client Operations and Scientific Data Strategy, Global Specimen Solutions, Inc.
Kate Dugan, Vice President, Client Operations and Scientific Data Strategy, Global Specimen Solutions, Inc.
 John "JJ" Spegele, Vice President, Business Solutions and Enterprise PMO, Covance Central Lab Services, Covance,
Inc.
John "JJ" Spegele, Vice President, Business Solutions and Enterprise PMO, Covance Central Lab Services, Covance,
Inc.
Pharmaceutical companies have collected vast amounts of data during a clinical trial, yet only ~20% of the data is used. Disparate data sources, unaligned data, and incomplete data sets prevent in-life interventions, lead to delays
in go to market, and can lead to non-compliance with regulations. In this presentation, we will discuss case studies that empowered translational medicine and clinical operations teams to make decisions that advanced drug development.
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
Evi Cohen, Vice President, Global Pharma & Life Sciences, Appian
10:35 CO-PRESENTATION: Incubating Digital Innovation Across the Clinical Trial Process
 Leyla Rich, IT Business Partner, Digital Health, Information Technology, Bristol-Myers Squibb
Leyla Rich, IT Business Partner, Digital Health, Information Technology, Bristol-Myers Squibb
 Erin Rossi, Category Manager, Clinical Core Technologies, Global Procurement, Bristol-Myers Squibb
Erin Rossi, Category Manager, Clinical Core Technologies, Global Procurement, Bristol-Myers Squibb
Our internal working group is executing a number of proof-of-concepts (POCs), pilots, and tests in 2017 to evolve the digital clinical trials landscape at Bristol-Myers Squibb aligned to our enterprise strategy and with a focus
on patient and site experience. These POCs focus on telemedicine, Quality of Life (QoL) metrics via wearables and sensors, and the use of digital technologies for adverse event reporting in Clinical Trials. As part of the session
we will also share the outcomes from these experiments.
11:00 BYOD: A Game-Changer for Sponsors, Sites and Patients
 Chris Watson, PhD, Director, Product Strategy, Digital Patient, ERT
Chris Watson, PhD, Director, Product Strategy, Digital Patient, ERT
Electronic clinical outcomes assessments (eCOA) are no longer just about how patients feel or function pre-approval. With recent technological advancements, eCOA has become a valuable tool that helps sponsors gain greater insight
into patient experiences post-approval – especially in site-less trials. A bring-your-own-device (BYOD) approach, coupled with wireless integration with mobile medical devices, opens up a whole new world of data collection
options for trial sponsors, sites and patients. In this discussion, we’ll share success stories and demonstrate why sponsors need to incorporate this approach.
11:25 Transition to Shared Sessions
11:35 Digital Trends Impacting Recruitment, Engagement and Retention
 Shwen Gwee, Head, Digital Strategy, Global Clinical Operations, Biogen
Shwen Gwee, Head, Digital Strategy, Global Clinical Operations, Biogen
Digital technology is connecting more people to clinical trials than ever before and at the same time the adoption of wearables as data collection devices in clinical trials is rising. The hope of streamlining trial operations,
patient recruitment and registration is real. What technolgies and approaches are having the greatest impact on recruitment, engagement and retention?
12:00 pm CASE STUDY: The Art and Science of Patient Engagement in the Digital Era – Learning from GSK PARADE Study
 Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
The ability to efficiently develop new medicines for patients with unmet needs is limited by the current model for clinical development. Although the emerging mHealth technologies have the potential to improve the conduct of clinical
trials, the successful implementation requires careful study design and patient engagement. Data and experiences from GSK PARADE study will be summarized, highlighting the learning and opportunities in this new area of clinical
development.
12:25 Mobile Clinical Trials: New Findings on Patient and Site Perspectives from the Clinical Trials Transformation Initiative
 Hassan Kadhim, Business Consultant for Clinical Operations, Boehringer Ingelheim Pharmaceuticals
Hassan Kadhim, Business Consultant for Clinical Operations, Boehringer Ingelheim Pharmaceuticals
This presentation will discuss qualitative and quantitative research conducted by the Clinical Trials Transformation Initiative to understand the perspectives of patients and site investigators of mobile clinical trials. Discussion
will include insights and advice from site investigators who have participated in trials that incorporate mobile devices to collect data for study endpoints.
12:50 PANEL DISCUSSION: Why Are There Barriers to the Adoption of Innovative Processes and Technologies at Sites?
Moderator: Jim Kremidas, Executive Director, Association of Clinical Research
Professionals (ACRP)
David Vulcano, Assistant Vice President & Responsible Executive for Clinical
Research, Hospital Corporation of America (HCA)
Sean Walsh, MBA, Chief Development Officer, Raleigh Neurology Associates
Beth Harper, MBA, Workforce Innovation Officer, Association of Clinical
Research Professionals (ACRP)
Many innovative technologies and process improvement initiatives are coming out at a rapid pace, whether from TransCelerate and other industry consortia, or from technology companies themselves. Which of these improvements
actually work? How can sites implement these more effectively? Why are there barriers to adoption, and how can the innovators better understand sites’ needs?
- Share sites’ perspective on the evolving clinical research landscape
- Discuss the reasons sites struggle with new processes and technology tools
- Determine ways to facilitate adoption
1:15 Closing Remarks
1:20 SCOPE Summit 2018 Adjourns
 Group Discounts Are Available! Special rates are available for
multiple attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for
multiple attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Marina Filshtinsky, M.D.
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech
Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1)
781.972.5456
E: rhandy@healthtech.com