Cambridge Healthtech Institute’s 10th Annual
Clinical Data Strategy and Analytics:
Enabling Data-Driven Clinical Trials
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
E-clinical technology is changing the landscape of the clinical research industry and healthcare IT in general. Digitalization of healthcare data, mobile data capture technologies, and cloud storage of data are a few of the main technological advances
that influence clinical research informatics. The technological advances have been coupled with novel data visualization solutions, and this powerful duo is helping to develop a new paradigm of data-driven clinical trials. Cambridge Healthtech Institute’s
10th Annual Clinical Data Strategy and Analytics conference is designed to bring together clinical research informatics experts to discuss the challenges and find solutions necessary to navigate and thrive in this
rapidly changing environment.
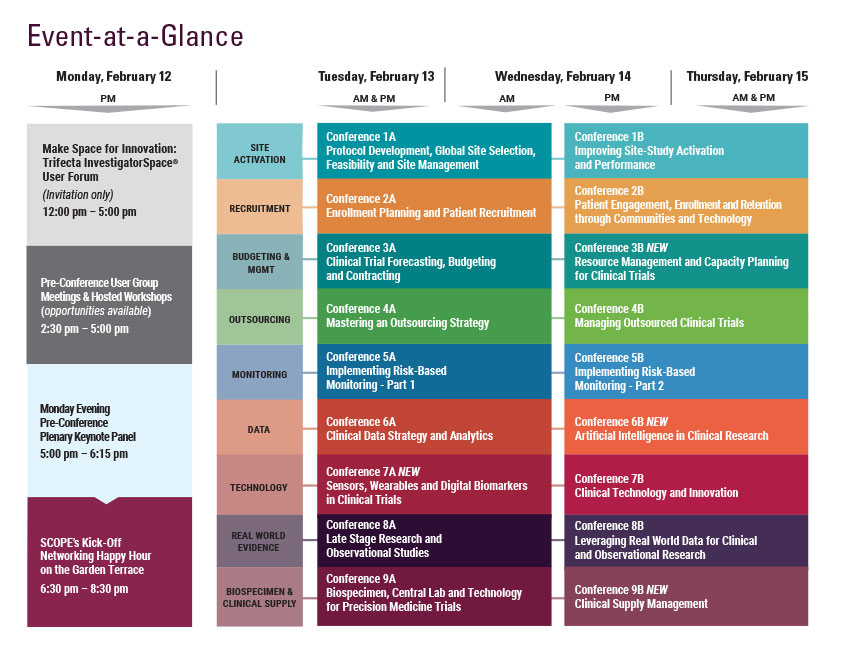
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Jaydev Thakkar, Product Innovation Lead, Amgen Digital Health
10:50 SPECIAL OPENING PRESENTATION: Integrated Technology Platforms and the Implications to Our Clinical Organizations
 Rehbar Tayyabkhan, Vice President, Global Data Strategies & Solutions, Global Clinical Operations,
Bristol-Myers Squibb
Rehbar Tayyabkhan, Vice President, Global Data Strategies & Solutions, Global Clinical Operations,
Bristol-Myers Squibb
As capabilities emerge with e-clinical technology platforms, esource, analytics, etc., clinical operations organizations have no choice but to adapt and realign our capabilities. This presentation will provide some context for some high-impact
emerging changes and share some practical approaches taken to realize the value from these technological advancements.
11:15 CO-PRESENTATION: Technology Innovations Transforming Clinical Research, Are We There Yet?
 Jaydev Thakkar, Product Innovation Lead, Amgen Digital Health
Jaydev Thakkar, Product Innovation Lead, Amgen Digital Health
Yan Chow, M.D., MBA, Medical Director, Digital Health, Amgen
Innovative digital technologies are starting to disrupt the highly regulated and conservative biopharmaceutical industry. We will examine the clinical and business drivers of this revolution, as well as its impact on how and why we conduct
research studies and clinical trials.
11:40 Implementing Large Change in a Large Organization
 Christine Buesnel, Director, Product Development, Biometrics, Roche
Christine Buesnel, Director, Product Development, Biometrics, Roche
This talk will inform the audience of a large system, process and people change for management of the medical data that Roche/Genentech undertook over a 3-year timeframe. I will discuss the scope of the change, the impact on the organization,
and the impact on the people. I will also address the lessons we learned and think are applicable to any large scale change in an organization.
12:05 pm CO-PRESENTATION: New Tufts Research: EDC Trends, Insights, and Opportunities
 Beth Harper, President, Clinical Performance Partners; Consultant, Tufts Center for the Study of Drug Development
Beth Harper, President, Clinical Performance Partners; Consultant, Tufts Center for the Study of Drug Development
 Richard Young, Vice President, Veeva Vault EDC, Veeva Systems
Richard Young, Vice President, Veeva Vault EDC, Veeva Systems
Hear new research from Tufts Center for the Study of Drug Development on current and evolving EDC practices across the drug development enterprise. Learn how seemingly minor decisions in one functional group can significantly impact overall
clinical trial timelines. We’ll also share where the industry is headed and ways to overcome key clinical data management challenges including protocol changes, source data verification, and more.
12:30 Session Break
12:40 Luncheon Presentation: Planning and Managing Effective Study Design Through EDC Data Analytics
 John Fontenault, COO, Omnicomm
John Fontenault, COO, Omnicomm
Recently, there has been raised awareness around over-engineered study designs driving study complexity, as well around potential cost reduction and improved data quality through effective use of Risk-Based Monitoring (RBM). Alignment of study
planning and design with study execution and data capture is imperative to support initiatives in these areas. This presentation will focus on insights gained from clinical and operational data analytics to improve EDC study design effectiveness
in support of these initiatives.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Christine Buesnel, Director, Product Development, Biometrics, Roche
2:05 Analytics to Drive Better Decisions in Clinical Development
 Angelique Hopkins, MPH, Associate Director, Clinical Trial Analytics, Business Insights and
Analytics, Bristol-Myers Squibb Company
Angelique Hopkins, MPH, Associate Director, Clinical Trial Analytics, Business Insights and
Analytics, Bristol-Myers Squibb Company
Integrated, predictive analytics can help drive R&D strategy and execution, with clear benefits to operational costs and long-term financial success. Analytics in trial planning and execution are often viewed as drivers of delay rather
than acceleration and analytics is rarely used effectively to drive decisions in planning. This presentation will discuss how embedding analytics into clinical development process can alleviate challenges and build trust with stakeholders
for faster, better decisions.
2:30 From the Trenches: Technical Strategies for Start-Up and Virtual Pharma/BioTech Companies
 Steven Sweeney, Vice President, Clinical Development Operations, Rodin Therapeutics
Steven Sweeney, Vice President, Clinical Development Operations, Rodin Therapeutics
This presentation will focus on the technology stack utilized for clinical development in the start-up and virtual pharma/biotech sector. It will include a review of popular start-up models and overlay considerations for the use of technology
to improve efficiency and vendor oversight and obtain scientific and medical insights.
2:55 Clinical Data Integration from Translational Modeling Using Machine Learning Approach
 Raj Bandaru, Senior Director, Data Sciences Strategy, Translational Medicine, Sanofi
Raj Bandaru, Senior Director, Data Sciences Strategy, Translational Medicine, Sanofi
Use of clinical data for translational modeling and trial simulation is key capability to transform the pharma industry to more data and model-driven drug development. At Sanofi, we have developed some elegant machine learning approaches to
integrate clinical study data and make it available for clinical research. This approach reduces the manual effort and thus enabling real time analytics and simulation of trial results.
 3:20 Visual Analytics for Medical Data Review: A Physician’s Perspective
3:20 Visual Analytics for Medical Data Review: A Physician’s Perspective
 Anthony Everhart, MD, FACP, Vice President, Medical Informatics, Covance
Anthony Everhart, MD, FACP, Vice President, Medical Informatics, Covance
The acceptance and adoption of new technology systems by physicians can be slow and is often met with resistance. Recent examples of this in clinical medicine include the challenges experienced when implementing electronic medical records
(EMR) and computerized physician order entry (CPOE). Perceived usefulness and perceived ease of use per Davis’ Technology Acceptance Model (1989) have an impact on physician acceptance or rejection of new technology.
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest
and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and
problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove such
concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice technology
and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Julie Smiley, Director, Product Strategy, Oracle Health Sciences
8:30 Harnessing Digital Technology and Big Data in Clinical Trials and Beyond
 Anthony Rowe PhD, Director, R&D IT Business Technology Leader, Immunology TA, Janssen
Anthony Rowe PhD, Director, R&D IT Business Technology Leader, Immunology TA, Janssen
Measuring physiological and activity-based parameters remotely and continuously via unobtrusive on/off-body sensors or smartphones has the potential to revolutionise our ability to monitor patients between clinic visits and develop objective
markers that track disease trajectory. How can we harness such advances in digital technology and big data analytics to enable more informative and efficient clinical studies and develop patient-centric digital solutions that improve
outcomes in the real world?
8:55 Digital Biomarker Implementation, Presentation and Comparability
 Amy Calvin, BS, MT (ASCP), MBA, Digital Strategy and Implementation, Advisor, Eli Lilly and Company
Amy Calvin, BS, MT (ASCP), MBA, Digital Strategy and Implementation, Advisor, Eli Lilly and Company
Over the past few years, data generation is beginning to take a new form. It’s moving from subjective to more objective, from episodic to contemporaneous, and is being generated through connected digital tools that can be used to
quantitatively explain or predict physiological, behavioral, and functional health measures and outcomes. These digital measures are known as digital biomarkers. This presentation utilizes learnings from pilot examples to examine the
implementation, presentation and comparability of digital biomarkers.
9:20 Wearables and Sensors Are Changing the Clinical Trial Process, but What’s the Return on Investment for This Dramatic Change in People, Process and Technology?
 Deborah Profit, Ph.D., Vice President, Otsuka Information Technology
Deborah Profit, Ph.D., Vice President, Otsuka Information Technology
The advent of wearables and sensors in clinical trials is changing the paradigm of trial designs, clinical teams, outsourcing practices, and ultimately patient engagement. However, what value does sensor/wearable data and these new
trial practices bring, and how quick is the return of investment to the various stakeholders? Otsuka Pharmaceutical Development and Commercialization, Inc. is on the cutting edge of the trial and technology reform, and will share
some critical learnings from “the road less traveled”.
9:45 CO-PRESENTATION: Driving Clinical Strategy & Optimized Design with RWD and Clinical Patient-Level Data
 Qin Ye, MD, Associate Principal, Global RWE Lead, ZS
Qin Ye, MD, Associate Principal, Global RWE Lead, ZS
 Jane Fang, MD, PhD, Head, Biologics, Research & Development Informatics, AstraZeneca/MedImmune
Jane Fang, MD, PhD, Head, Biologics, Research & Development Informatics, AstraZeneca/MedImmune
Historical clinical trial and real-world data hold immense potential in sharpening R&D strategy, optimizing trial design and streamlining operational planning. This data-driven approach has become common practice for many in the
pharmaceutical industry, but there are still hurdles to overcome. For some, the lack of efficient and timeline approach as well as tangible use cases leads to poor adoption and impact. In this presentation we’ll share how
MedImmune leveraged an integrated framework to enable seamless data-driven decision making and process transformation within clinical program planning.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Venkat Sethuraman, Associate Principal, ZS
11:15 Data Integration Solutions: A Case Study of CSL Behring’s Evolution on Managing Clinical Data
 Steve Carr, Director, Data Management, Coding, CSL Behring
Steve Carr, Director, Data Management, Coding, CSL Behring
CSL Behring had three new molecular entity approvals in the last two years for rare diseases to treat factor eight and nine deficiencies in hemophilia, along with hereditary angioedema. How does a company go from running small rare
disease studies to delivering a Phase III CV mega-trial? We will discuss how we have spent the last two years preparing to start this study in the first half of 2018.
11:40 CO-PRESENTATION: Randomization Authorization Flow (RAF): It’s Not Just about Meeting Eligibility Criteria
 Jody Goldstein, Senior Clinical Project Manager, Center for Human Experimental Therapeutics, University of
Rochester
Jody Goldstein, Senior Clinical Project Manager, Center for Human Experimental Therapeutics, University of
Rochester
 Susan Bennett, Senior Clinical Data Manager, Center for Human Experimental Therapeutics, University
of Rochester
Susan Bennett, Senior Clinical Data Manager, Center for Human Experimental Therapeutics, University
of Rochester
RAF is a review and approval process of predetermined key data points and eligibility criteria by a single point of contact (medical monitor) prior to randomization. The RAF process helps to ensure enrollment of the appropriate study-specific
patient population. This gestalt review takes into account critical elements not necessarily covered by the eligibility criteria. Looking for subtle (subjective) differences between patients upfront ensures meeting the primary
outcomes of the study.
12:05 pm Session Break
12:10 BRIDGING LUNCHEON CO-PRESENTATION: Centralizing Data to Address Imperatives in Clinical Development
 Karim Damji, Senior Vice President, Products, Solutions & Marketing, Saama Technologies
Karim Damji, Senior Vice President, Products, Solutions & Marketing, Saama Technologies
 Amit Gulwadi, Executive Director, Analytics-Patient Engagement/Recruitment, Celgene
Amit Gulwadi, Executive Director, Analytics-Patient Engagement/Recruitment, Celgene
With the deluge of structured, unstructured, and syndicated data, the use of varied data for targeted outcomes remains difficult, despite increased industry efforts to address the issue. New technologies are federating the
ability to leverage analytic-ready data for innovations in clinical development and drug commercialization. With the application of clinical data-as-a-service and meta-data core, centralized clinical data lakes have the
power to improve data quality, evidence generation, and time-to-insights.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Marina Filshtinsky, M.D.
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute
(CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com