Cambridge Healthtech Institute’s Inaugural
Clinical Supply Management
Effective Tracking, Managing and Distributing Clinical Supplies
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
Successful, patient-centric clinical trials depend upon streamlined clinical trial supply processes that ensure that the study drug is properly handled and delivered to the right patient whether at the trial site, pharmacy or in their home. Cambridge
Healthtech Institute’s Inaugural Clinical Supply Management conference offers case studies and practical solutions from across pharma focusing on effective clinical supply management from handling the study drug to direct-to-patient
distribution.
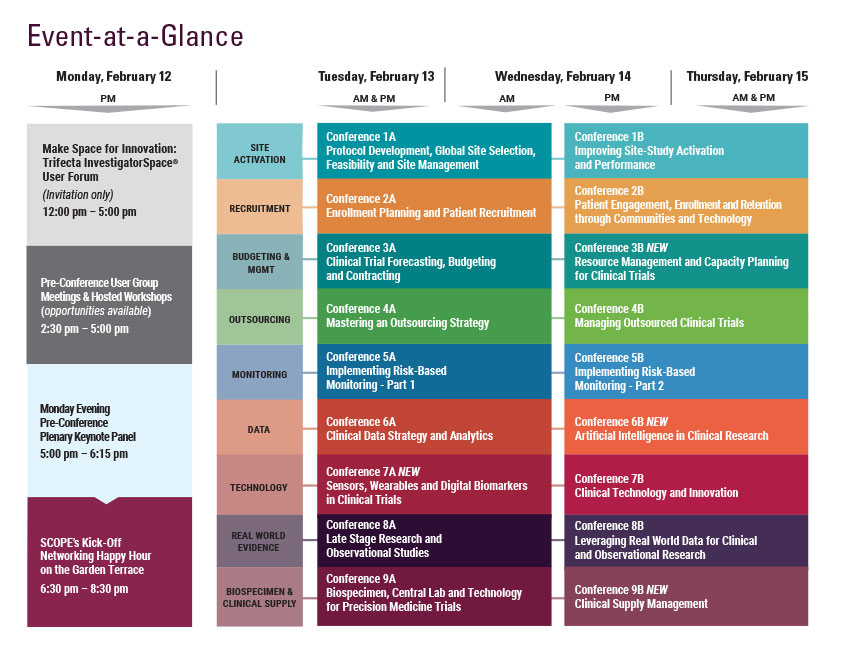
Wednesday, February 14
11:30 am Registration Open
 12:10
pm Luncheon Presentation: Case Study: How to Select and Deploy a Forecasting Solution and Measure its ROI Post-Implementation
12:10
pm Luncheon Presentation: Case Study: How to Select and Deploy a Forecasting Solution and Measure its ROI Post-Implementation
 Oliver Cunningham, Director, Client Enablement, Bracket
Oliver Cunningham, Director, Client Enablement, Bracket
This presentation will discuss how a top 20 pharmaceutical company was able to save over $25M on a single clinical program within 12 months of implementing a new forecasting software that drives demand planning and supply chain optimization. Oliver will
present the case-study, review the Forecasting tool’s specific features, and discuss how integrating IRT and forecasting creates a dynamic planning model feeding of actual data.
12:50 Coffee and Dessert Break in the Exhibit Hall
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
Doug Meyer, MBA, RPh, Associate Director, Clinical Drug Supply, Biogen, Inc.
4:05 Building an Effective End-to-End Cold Chain Supply Management Process
 Doug Meyer, MBA, RPh, Associate Director, Clinical Drug Supply, Biogen, Inc.
Doug Meyer, MBA, RPh, Associate Director, Clinical Drug Supply, Biogen, Inc.
This presentation will discuss: 1. What tools and technologies can you leverage to protect the product and avoid costly temp excursions? 2. How can you build efficient processes that ensure drug is always available for patient dosing? 3. What strategies
should you adopt in your stability program to enable an efficient and scalable supply chain for temperature sensitive products?
4:30 Is It Time for the Industry to Develop Consensus-Based, Standardized Clinical Supply Performance Metrics?
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
 4:55 Patient-Centric
Services: Points to Consider for Your Trial and How to Avoid Challenges
4:55 Patient-Centric
Services: Points to Consider for Your Trial and How to Avoid Challenges
 Michael Macneir, Director, Commercial Operations, Marken
Michael Macneir, Director, Commercial Operations, Marken
Patient-centric services are growing at incredible rates throughout the industry. Complexity within the supply chain is also growing as the sensitivity and value of products grows. Explore critical points to consider to protect your drug products when
setting up a DTP trial.
 5:10 Managing
Complex Supply Challenges in Oncology Studies
5:10 Managing
Complex Supply Challenges in Oncology Studies
 Phil Woodson, Senior Software Engineer, 4G Clinical
Phil Woodson, Senior Software Engineer, 4G Clinical
5:25 Achieving Accurate Forecasts and Minimizing Delays in Clinical Trials
 Paul Larochelle, PharmD, RPh, Senior Manager, Clinical Asset Planning, Specialty Therapeutic Area, Clinical Drug Supply, Biogen
Paul Larochelle, PharmD, RPh, Senior Manager, Clinical Asset Planning, Specialty Therapeutic Area, Clinical Drug Supply, Biogen
This presentation will 1. Discuss the importance of accurate forecasting in clinical trials and associated challenges, 2. Review how accuracy is driven by the strength of data points/information provided to supply professionals, 3. Determine ways to enhance
relationships with partners to manage unanticipated variations and minimize delays, 4. Examine how tools can help us better manage our studies and supplies
 5:50 Reception Hosted by Cognizant Technology Solutions
5:50 Reception Hosted by Cognizant Technology Solutions
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation,
we will go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
8:35 Supply Chain Integrity; Technology Tools for Tracking and Tracing Clinical Supplies
 Tom Skiendzielewski, Associate Director, Clinical Supply, Shire
Tom Skiendzielewski, Associate Director, Clinical Supply, Shire
This presentation will discuss: 1. Benefits of ERP system application in Clinical Supply Management (in-source vs. out-source), 2. Scoping your IRT to optimize benefit, reduce risk, and increase compliance, 3. Comprehensive Technology-Based Time
Out of Environment (TOE) Management, 4. Analyzing RFID utilization in the Clinical Supply Chain, and 5. The Last Link of the Clinical Supply Chain – Site Level Management
9:00 IRT Considerations for Managing Clinical Supplies
Carol Lee, Associate Director, Clinical Drug Supply, Logistics, & IRT, Regeneron
This presentation will discuss: 1. Exploring methods through which IRT technology can improve clinical supply logistics, 2. Pinpointing how IRT technology can be set up to improve your logistical planning, 3. Exploring the benefits of creating
your own in-house IRT system versus using a base-line vendor model, 4. Recognizing the value of IRT in clinical supply ordering and distribution, and 5. Emphasizing ways through which IRT systems can become more effective for your different
trials.
9:25 IRT Data Integration Provides Big Benefits
Aaron Harlett, Director, Supply Chain Systems and Related Services, Eli Lilly and Company
It’s obvious an IRT makes conducting a clinical trial more efficient and secure; from study drug forecasting and distribution to treatment group assignment and blinding. But using an IRT can provide benefits in many other aspects of trial
execution. IRT allows near real-time data capture that can seamlessly integrate with multiple systems and functions while safeguarding sensitive data through automation. And combining data from an IRT with patient data from other systems can
allow information alignment and facilitate reconciliation. And sharing a consistent data flow from an IRT can reduce vendor cost and play a critical role in bringing an asset to market in less time.
 9:50 Eliminating Peepholes into Blinded Clinical Trials
9:50 Eliminating Peepholes into Blinded Clinical Trials
 Gayle Flynn, MA, Director, Cognizant Life Sciences Consulting, Interactive Response Technology Practice, Cognizant
Gayle Flynn, MA, Director, Cognizant Life Sciences Consulting, Interactive Response Technology Practice, Cognizant
As Clinical Ops professionals, we consider conducting blinded clinical trials a core competency. Then one day unexpectedly, we receive an email that someone believes they can tell the treatment allocation of patients in a trial that seemingly
was not at risk for unblinding. This session will review the points to consider when setting up studies to ensure the blind is adequately maintained throughout the clinical supply chain.
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
10:35 FEATURED PRESENTATION: Patient Centered Clinical Trial Material Design and Delivery
 Janelle Sabo, Pharm.D., RPh, MBA, Senior Group Director, Product Delivery, Eli Lilly
Janelle Sabo, Pharm.D., RPh, MBA, Senior Group Director, Product Delivery, Eli Lilly
We will explore areas for patient-centered design and delivery in CT material, including formulation/drug product, kitting, delivery and technology. Practical current examples and inspirational goals will both be discussed to frame where we are
today but where the future opportunities lies within clinical supplies.
 11:00 Research Reveals: RTSM Frustrations, Expectations, and Future Predictions
11:00 Research Reveals: RTSM Frustrations, Expectations, and Future Predictions
Greg Jones, Enterprise Strategy Architect, Product Strategy, Oracle Health Sciences
A recent survey of more than 250 clinical operations professionals conducting randomized trials reveals frustrations with current systems, must-have technology innovations and future predictions for Randomization and Trial Supply Management In
this session: 1) Hear what 92% of clinical study teams must do more than once in a randomized trial 2) Understand the current state and future direction of RTSM technology 3) Discover how the new capability platform approach will provide huge
benefits for RTSM.
11:30 Brief Session Break
11:35 The Near-Term Viability and Benefits of eLabels for Clinical, Sites and Patients
 Jodi Smith-Gick, RPh, Senior Advisor, Product Delivery and Supply, Eli Lilly and Company
Jodi Smith-Gick, RPh, Senior Advisor, Product Delivery and Supply, Eli Lilly and Company
This session will speak to the options and benefits of utilizing eLabeling to enhance site efficiency and enhance patient centricity. Specifically discussed will be approaches, considerations when planning a study, and potential add-on technologies
which can further improve productivity at sites. Specific feedback received from patients and sites on these concepts will be shared. In addition, this session will de-bunk some of the misconceptions around the near-term viability for an eLabel
solution.
12:00 pm PANEL DISCUSSION: Direct-to-Patient Distribution: Meeting the Patient’s Needs
Moderator:Paddy Hanlon, Vice President, Global Key Accounts, Marken
Panelists: Gerald Finken, CSO/Founder and Innovator, Clinical Supplies Management
 Janelle Sabo, Pharm.D., RPh, MBA, Senior Group Director, Product Delivery, Eli Lilly
Janelle Sabo, Pharm.D., RPh, MBA, Senior Group Director, Product Delivery, Eli Lilly
As the pharma industry moves towards more patient-centric initiatives for clinical trials, direct-to-patient distribution is growing in popularity, but many challenges still remain. Topics discussed in this panel include: 1. Investigator and site
buy-in and support for direct-to-patient initiatives, 2. Logistical, cost and regulatory considerations; and 3. Challenges with patient handling of IMPs.
12:50 Closing Remarks
1:00 SCOPE Summit 2018 Adjourns
 Group Discounts Are Available! Special rates are available for multiple attendees from
the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees from
the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
Lee Yuan
Conference Director
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com