Cambridge Healthtech Institute’s 2nd Annual
Mastering an Outsourcing Strategy
Making Meaningful Changes to Outsourcing Strategy & Vendor Selection
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
Understanding outsourcing needs and optimizing the selection process of vendors lays the foundation for an efficient, cost-effective clinical trial. Cambridge Healthtech Institute’s 2nd Annual Mastering an Outsourcing Strategy conference provides a new perspective on the vendor selection process and challenges attendees and speakers to reimagine the standard vendor selection process for improved efficiency on both the Sponsor and Vendor side of the relationship. The 2018
program focuses on case studies and interactive discussion on outsourcing strategy, the RFP and bid defense process, vendor selection, as well as contracting with outsourced partners and vendors, including sites, CROs, suppliers, and other vendors.
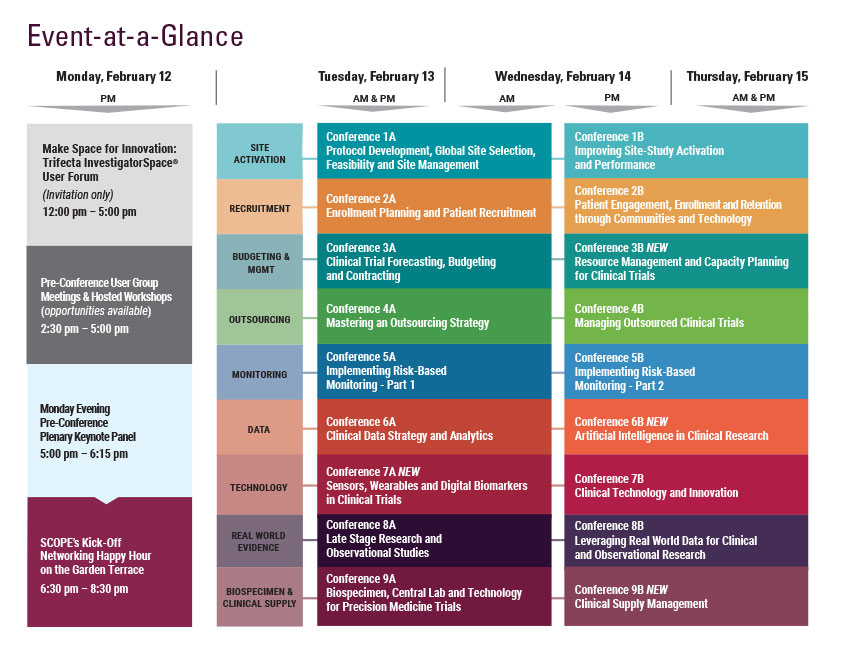
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Charlotte French, Executive Director, Portfolio Relationship & Sourcing Management, Medical and Development, Astellas
10:50 Why Outsourcing Site Payments Make Sense
Brett Kleger, CCO, DrugDev
11:15 Sourcing Model Considerations
 Ratan Ratnesh, Director & Head, Clinical Outsourcing, Otsuka
Ratan Ratnesh, Director & Head, Clinical Outsourcing, Otsuka
This presentation will focus on an approach to assessing and developing which sourcing model is right for your organization. We will define what a Sourcing Model is and how companies come to arrive at their current state. Then we will review
sourcing options, considerations, and implications both on a functional level and organizational level. We will review the importance of assessing core competencies as well as a thorough market analysis. Then conclude with how all the
pieces fit together to form a sourcing model that best fits your organization.
 12:05
pm Trends in Outsourcing: The Talent Dynamic
12:05
pm Trends in Outsourcing: The Talent Dynamic
 Mark Lanfear, MS, CCRA, Vice President and Global Practice Lead, Life Sciences Solutions, Kelly Outsourcing and Consulting
Group (KellyOCG)
Mark Lanfear, MS, CCRA, Vice President and Global Practice Lead, Life Sciences Solutions, Kelly Outsourcing and Consulting
Group (KellyOCG)
In an industry of disease and discovery, we are tasked with making the quality of patient care better, and workforce trends that impact outcomes are critical. Regulation, therapeutic focus, the dynamic world economy, and exponential changes
in the talent market are making it essential not only to consider your future workforce needs, but much more deliberately institute a strategy of how to meet and manage workforce resources and capacity over the next five years.
12:30 Session Break
 12:40 LUNCHEON
CO-PRESENTATION: Protocol Complexity and Strategic Outsourcing
12:40 LUNCHEON
CO-PRESENTATION: Protocol Complexity and Strategic Outsourcing
Tess Gilbert, Consultative Solutions, Clinical Trial Optimization Solutions (CTOS), IQVIA
Kyle Holen, Head, Drug Development Center, AbbVie
The most foundational element of expectation setting between Sponsor, CRO and Site, is many times the most uncertain. As protocols become more complex, the impact can be strain on sponsor/CRO relationships. A recent survey conducted with Sites,
Sponsor and CROs tells a story of uncertainty, and lack of evidence to support complexity assessments. Join us for a panel discussion to review best practices in complexity assessment to generate fair and predictive expectations in strategic
outsourcing.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Charlotte French, Executive Director, Portfolio Relationship & Sourcing Management, Medical and Development, Astellas
2:05 A Nimble, Rapid Approach to the RFI to Bid Defense Process
 Craig Coffman, Executive Director, Clinical Business Operations & Outsourcing, Nektar Therapeutics
Craig Coffman, Executive Director, Clinical Business Operations & Outsourcing, Nektar Therapeutics
The RFI to bid defense process is notoriously time consuming and resource intense. One biopharmaceutical company presents their streamlined strategy for efficiently moving through the RFI to bid defense process while involving key stakeholders
at every step and remaining nimble without sacrificing quality.
2:30 PANEL DISCUSSION: Request for Information (RFI) to Bid Defense – How Do Pharma and CROs Obtain the Most Value during This Process?
Moderator:
 Charlotte French, Executive Director, Portfolio Relationship &
Sourcing Management, Medical and Development, Astellas
Charlotte French, Executive Director, Portfolio Relationship &
Sourcing Management, Medical and Development, Astellas
Panelists:
 Christopher Rull, Principle Consultant, CR Consulting, LLC; Former Vice President, Head of Business Development
& Account Management, UBC
Christopher Rull, Principle Consultant, CR Consulting, LLC; Former Vice President, Head of Business Development
& Account Management, UBC
Jeff Van Noy, Vice President, Global Proposal Development and Business Information, ICON
Today this is a very time consuming and resource intense activity at both Pharma and CROs. This panel discussion will be focused on how we jointly become more innovative in approaching these activities. We will explore:
- How to develop the most effective RFI to deliver the key information required to determine which CROs are short-listed for the Bid Defense meeting
- How Pharma/CRO determine the participants at the Bid Defense meeting
- What are the key deliverables for a CRO at a Bid Defense meeting?
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of
interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation
and problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove
such concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice
technology and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Mark Lanfear, MS, CCRA, Vice President and Global Practice Lead, Life Sciences Solutions, Kelly Outsourcing and Consulting Group (KellyOCG)
8:30 Outsourcing versus Procurement: Is There a Difference?
 Charlotte French, Executive Director, Portfolio Relationship & Sourcing Management,
Medical and Development, Astellas
Charlotte French, Executive Director, Portfolio Relationship & Sourcing Management,
Medical and Development, Astellas
Today it can be difficult to navigate the various functions within Pharma, in particular relating to the roles and responsibilities of Outsourcing functions versus Procurement functions. In some instances, these are combined, but are
there differences? And, if so, what are these? We will explore the advantages and disadvantages of separating these two functions within Pharma and review differences in the skill set that they should offer to an organization.
Additionally, we will review how these functions work together, or in some instances, incorporate the vendor oversight responsibilities that are required under ICH E6. We will also discuss CRO’s response to the ICH E6 guidelines
when providing third party contracting and oversight for additional services.
8:55 Choosing & Maintaining the Best Suppliers/Partners
 Carrie Lewis, MS, Associate Director, Clinical Operations, Lupin Research, Inc.
Carrie Lewis, MS, Associate Director, Clinical Operations, Lupin Research, Inc.
This presentation will discuss how to select the best Supplier/Partner for your company needs. This will include a case study of how Lupin set-up their initial Governance with all CROs at the table. Discussion will also include when
to select a niche supplier over preferred vendor and the process. Lastly, the talk will discuss how to maintain relationships with preferred vendors.
9:20 Contracting with CROs to Optimize Working Relationship and to Guarantee On-Time Study Starts
 Richard O’Hara, Associate Director, Clinical Outsourcing, Endo Pharmaceuticals
Richard O’Hara, Associate Director, Clinical Outsourcing, Endo Pharmaceuticals
This presentation will focus on the best strategies to ensure a smooth study start from a contracting perspective. It will address agreement construction and helpful terms. Also addressed will be optimal lead times as well as governance
and communication strategies.
9:45 Three Key Elements to Operationalizing CRO Oversight: A Sponsor’s Story
 Rick Morrison, Co-Founder and President, Comprehend Systems
Rick Morrison, Co-Founder and President, Comprehend Systems
Learn how a leading sponsor revolutionized its relationship with its CROs. Understand the best practices they put in place to continuously manage study quality and achieve milestones on-time and on-budget across their portfolio of
trials. This, combined with real-time data delivery, enabled them to expand their portfolio of trials and build new relationships with more CROs, all at the same rapid pace of business.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Anca Copaescu, CEO, Strategikon Pharma
11:15 PANEL DISCUSSION: Resource Allocation and Its Effect on Contracting between CROs and Sponsors
 Greg Skalicky, Executive Vice President and General Manager, Syneos Health
Greg Skalicky, Executive Vice President and General Manager, Syneos Health
 Ratan Ratnesh, Director & Head, Clinical Outsourcing, Otsuka
Ratan Ratnesh, Director & Head, Clinical Outsourcing, Otsuka
 Tara Dubois, MBA, Head, Clinical Trial Cost Management, Business Operations, Pfizer
Tara Dubois, MBA, Head, Clinical Trial Cost Management, Business Operations, Pfizer
Daniel Smith, Vice President, Global Solutions, Kelly OCG
Procurement, contracting, and clinical operations teams need transparency with their CRO partners in order to properly understand a CRO’s allocation of resources and costs, especially when contracting key deliverables. This panel
will address, from the Sponsor’s and CRO’s perspective, resource allocation considerations during the contracting process: potential challenges, what CROs wish pharma knew, and pitfalls to avoid.
12:05 pm Session Break
 12:10 Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
12:10 Bridging Luncheon Presentation: Data and Insights into Site Operations Allow for Better Forecasting and Budgeting of Trials
 Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
Noelle Gaskill, MBA, Vice President, Research Operations, SignalPath, LLC
By leveraging site level tooling to surface nearly real-time operational metrics; sponsors and CROs are better equipped to plan for costs associated with individual sites, manage ongoing trial costs and project future budgets.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
Lee Yuan
Conference Director
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1)
781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com