Cambridge Healthtech Institute’s 11th Annual
Enrollment Planning and Patient Recruitment:
Strategic Enrollment Planning, Data-Driven Recruitment and Forecasting, and Central Campaign Management
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
Patient recruitment and up-front enrollment planning are critical to drug development programs. Patient recruitment if not adequately planned for can extend your development timeline by a number of years. Retention of patients throughout the life of a
clinical trial is essential in order have complete data sets for your analysis and subsequent filings. In order to optimize both you have to have a plan. CHI’s 11th Annual Enrollment Planning and Patient Recruitment will
cover the topics one should consider when drafting and strategically implementing a patient recruitment plan for a clinical development program.
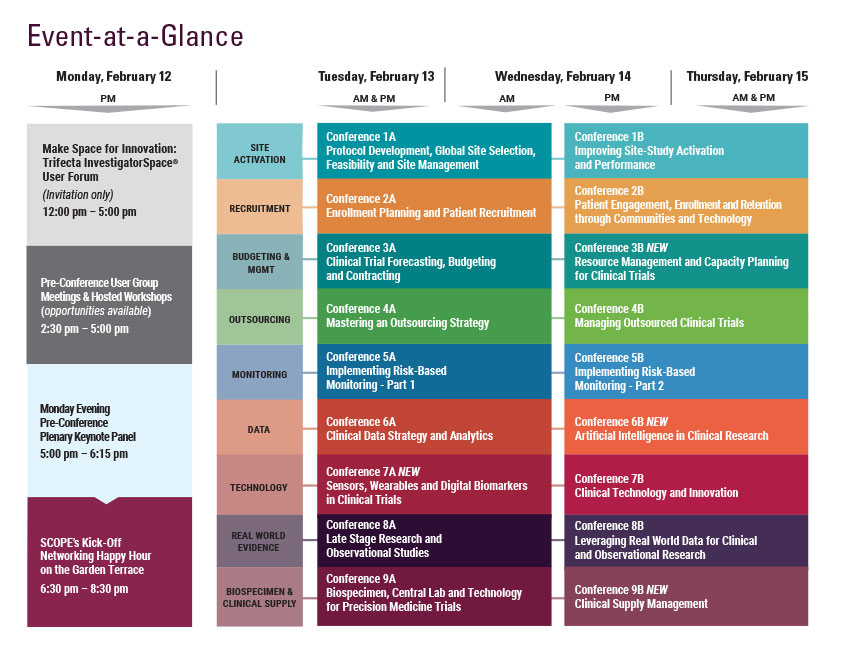
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Marisa Rackley, Director, Clinical Research Operations, Vertex Pharmaceuticals, Inc.
10:50 Leveraging Data and Analytics for Enrollment Planning and Trial Execution
 Susan Griffing, Vice President, Global Head Country Clinical Operations, Roche
Susan Griffing, Vice President, Global Head Country Clinical Operations, Roche
Clinical trials are still taking longer than planned and costing companies more than predicted. Of the challenges faced in trial execution, enrollment is still a major bottleneck for research. This presentation will talk to industry trends
in this area and specifically how we are leveraging data to make decisions around operations.
11:15 Patient Voice Plans in R&D: Improving the Patient Experience and Clinical Trial Success
 Katherine Capperella, Global Patient Engagement Leader, Janssen
Katherine Capperella, Global Patient Engagement Leader, Janssen
Janssen has employed a focused approach to incorporating patient insights into clinical trial design. Working together, commercial and R&D colleagues gather patient feedback and modify clinical trials to improve the patient experience,
leading to better recruitment and retention. This presentation will include case studies and an example of how patient engagement is being measured.
11:40 Bringing the Patient Voice and Community into the Drug Development Process
 Francis Kalush, Ph.D., Health Programs Coordinator, Professional Affairs and Stakeholder Engagement (PASE), CDER,
FDA
Francis Kalush, Ph.D., Health Programs Coordinator, Professional Affairs and Stakeholder Engagement (PASE), CDER,
FDA
FDA’s Professional Affairs and Stakeholder Engagement at CDER has been working with all stakeholders: patients/patient advocates, health professionals and industry to bring the patient voice and perspective into the drug development
and approval process. The goal would be to continue the support of novel therapies that directly address the need of patients living with diseases. Learn to effectively engage with FDA. Understand how to bring the voice of the patient
into the drug approval process.
12:05 pm Learning from Other Industries
 Ivor Clarke, CEO, SubjectWell
Ivor Clarke, CEO, SubjectWell
This presentation focuses on the root causes of this industry’s challenges in patient outreach and engagement. Then we’ll take a look at how other industries have solved similar problems and what we can apply to patient recruitment.
12:30 Session Break
12:40 Luncheon Co-Presentation: Navigating Clinical Research from the Perspective of the Patient: A Journey in Humility
 Robert Loll, Senior Vice President, Business Development & Strategic Planning, Praxis Communications, LLC
Robert Loll, Senior Vice President, Business Development & Strategic Planning, Praxis Communications, LLC
 T.J. Sharpe, Patient Advisor, Starfish Harbor LLC
T.J. Sharpe, Patient Advisor, Starfish Harbor LLC
Our industry is dependent upon the good intentions of ordinary volunteers subjecting themselves to protocols that regularly include extraordinary procedures. Patients must understand their diagnosis, acquaint themselves to available trial/treatment
options, comprehend the medical impact of their decisions, and choose a therapy – typically without a background in healthcare. This interactive session will walk the audience through the patient journey, focusing on treatment/trial
selection from a newly-diagnosed patient with a potentially life-threatening disease perspective.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Jill Johnston, President, Clinical Services Organization, WCG
2:05 Pharma Bands Together to Activate a New Audience: HealthCare Providers
 Joe Kim, MBA, Senior Advisor, Clinical Innovation, Eli Lilly and Company
Joe Kim, MBA, Senior Advisor, Clinical Innovation, Eli Lilly and Company
It’s no secret that attempts to inspire and enable health care providers to refer their patients into clinical trials have often failed. Reasons for this have been documented in peer reviewed literature and felt first hand by study teams
in real world campaigns. Come learn about a key component of TransCelerate’s Clinical Research Awareness and Access workstream where they look to go beyond incentive models and skill building. Learn about a unique photojournalism
campaign that seeks to activate the hearts of HCPs in a way that will make them believe in the benefit of research for patients, tomorrow and today.
2:30 Data-Driven Patient Recruitment with Real World Data at Roche pRED
 Liping Jin, Data-Driven Recruitment Lead, Pharmaceutical Research & Early Development, Roche Innovation Center
New York
Liping Jin, Data-Driven Recruitment Lead, Pharmaceutical Research & Early Development, Roche Innovation Center
New York
With the increasing use of Real World Data (RWD) in the pharma industry, Data-Driven Recruitment (DDR) team at Roche Pharm Research & Early Development (pRED) would like to share our experience of integrating RWD (e.g. insurance claims,
EMR) with trial metrics data to optimize study protocol design and target patient recruitment strategy. While the team has received positive feedback from our business partners (translational medicine, clinical program teams, and study
leaders) we would like also to share the challenges to expanding the effort in broader US and international settings.
2:55 PANEL DISCUSSION: Innovation in Recruitment Is Not a 4-Letter Word
 Kevin Hudziak, Innovation Lead, Clinical Innovation, Eli Lilly and Company
Kevin Hudziak, Innovation Lead, Clinical Innovation, Eli Lilly and Company
 Mark Springer, Project Lead, Clinical Innovation, Eli Lilly and Company
Mark Springer, Project Lead, Clinical Innovation, Eli Lilly and Company
 Taylor Wong, LRL Procurement, Medical and Regulatory, Eli Lilly and Company
Taylor Wong, LRL Procurement, Medical and Regulatory, Eli Lilly and Company
 Rahlyn Gossen, Founder & Principal, Rebar Interactive
Rahlyn Gossen, Founder & Principal, Rebar Interactive
Patient recruitment methods and execution have become stale. In the typical full-service model, a sponsor chooses one full-service supplier, who may not have expertise in all aspects of digital patient recruitment. To innovate in this
space, Lilly Clinical Innovation sought to determine if creating a new patient recruitment sourcing solution would benefit the digital patient recruitment ecosystem. By analyzing each aspect of the ecosystem, we looked to identify
the best in class vendors for each aspect of digital patient recruitment (e.g., creative, outreach, microsites, etc.) using Lilly TrialGuide as the centerpiece. This panel will examine the innovation and will represent the voices of
Clinical Innovation, Research Procurement, and a supplier involved in the process. Our process for determining selected suppliers will be explored including defining the “Pitch Match” method for determining final suppliers.
Communication, processes, challenges, best practices, and insights from all sides of the table will be explored and audience participation is encouraged.
3:20 Real World Data Meets Real World Evidence in Patient Recruitment and Engagement
 Bonnie Brescia, Founding Principal, BBK Worldwide
Bonnie Brescia, Founding Principal, BBK Worldwide
Today we have access to multiple health databases containing myriad data points that can be integrated, correlated and mined. The expectation is that these data will help researchers to better define target patient populations, improve
protocol design, and enhance site selection. But will these efforts advance patient recruitment and retention? Using data from multiple global trials, we explore RWE pointing to the decisive role of support and engagement programs
in recruiting and retaining study participants.
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of
interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation
and problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove
such concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice
technology and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Kevin Hudziak, Innovation Lead, Clinical Innovation, Eli Lilly & Company
8:30 Deploying Patient Recruitment Websites: Behind the Scenes in Big Pharma
 Paul Whitehead, Ph.D., Head, Early Development Workflows, Roche
Paul Whitehead, Ph.D., Head, Early Development Workflows, Roche
This talk will focus on the efforts that go into getting a patient recruitment website progressed from a study team request to production deployment. There are many factors to consider when deploying a new trial recruitment website
in a global organization, these will be discussed, and strategies to reduce the effort will be presented.
8:55 Increasing Enrollment While Reducing Sites
Daniel Brunwasser, Associate Director, Marketing Operations, Consumer Marketing, Acurian
After decades of delayed trials, sponsors still face slow site setup and poor patient enrollment completing clinical studies on-time and on-budget. The traditional site-based model suggests that the more sites activated, the more patients
and faster completion time happen. These delays prove that model lacks the productivity to move the chains, threatening time-to-file and time-to-market. Sponsors are now following a new model of enrollment and budget certainty
built on reducing sites/countries for faster patient enrollment.
9:20 Lessons Learned from Rare Disease Trials: Community, Engagement, Recruitment and Retention
 Marisa Rackley, Director, Clinical Research Operations, Vertex Pharmaceuticals, Inc.
Marisa Rackley, Director, Clinical Research Operations, Vertex Pharmaceuticals, Inc.
Although late-phase costs of conducting orphan drug development are smaller when compared to non-orphan drugs, rare disease clinical trials encounter many challenges in patient recruitment and retention. What lessons can experts in
trial planning and operations learn, whether they are running rare or non-rare disease trials, and apply to improve engagement, recruitment and retention?
9:30 SCOPE’s 2018 Participant Engagement Award
Designed to inspire innovation and change in how the industry communicates with participants in the fields of recruitment and retention in clinical trials, this award embodies the values and personal accomplishments of Jerry Matczak,
who sadly passed away soon after receiving the 2017 award. We dedicate this award to Jerry in the hopes that it will serve as a reminder of his ideals and accomplishments. Join us as Sites, CRO’s, e-Patient Advisors,
Agencies, Start-Ups, and Sponsors present their elevator pitch on how they have raised the bar in engagement with potential study participants. This light hearted approach to a serious subject allows attendees a look into the
latest innovations that put study participants first. Proud to announce 2018 Finalists:
Greenphire - Hope for Houston
Clara Health - Breakthrough Crew: Demystifying Clinical Trials Alongside Patient Advocates
GRYT Health Inc - Empowering patients to connect
with peers and new treatment options
Brite Health - Improving patient experience, compliance, and retention using data-driven technology
mProve Health with Pfizer - Patient Engagement Hub
Learn More
Event Designers:
 Kelly McKee, Advisor, Clinical Innovation, Eli Lilly and Company; Co-Creator of the SCOPE Participant
Engagement Award
Kelly McKee, Advisor, Clinical Innovation, Eli Lilly and Company; Co-Creator of the SCOPE Participant
Engagement Award
 David Sall, President & CEO, Patient Enrollment Advisors; Co-Creator of the SCOPE Participant
Engagement Award
David Sall, President & CEO, Patient Enrollment Advisors; Co-Creator of the SCOPE Participant
Engagement Award
 Jeri Burtchell, Director, Patient Initiatives, HealthiVibe, LLC
Jeri Burtchell, Director, Patient Initiatives, HealthiVibe, LLC
 Shwen Gwee, Head of Digital Strategy, Global Clinical Operations Biogen
Shwen Gwee, Head of Digital Strategy, Global Clinical Operations Biogen
 Angela Radcliffe, GM, Clinical Trial Solutions, PulsePoint
Angela Radcliffe, GM, Clinical Trial Solutions, PulsePoint
 Micah Lieberman, Executive Director, Conferences, Cambridge Healthtech Institute (CHI)
Micah Lieberman, Executive Director, Conferences, Cambridge Healthtech Institute (CHI)
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Rick Ward, Vice President, Commercial Operations, Trifecta
11:15 CO-PRESENTATION: Creating A Successful Patient Opinion Leader Steering Committee
 Lynn Hagger, Ph.D., Patient Engagement Director, Respiratory, INA & CVMD, Global Medical Affairs,
AstraZeneca
Lynn Hagger, Ph.D., Patient Engagement Director, Respiratory, INA & CVMD, Global Medical Affairs,
AstraZeneca
 Abbe Steel, CEO, HealthiVibe, LLC
Abbe Steel, CEO, HealthiVibe, LLC
As pharma struggles to turn the concept of patient engagement into more than just a feel-good catchphrase, there is a growing trend to partner with patients in a more meaningful way to impact development throughout the drug
life cycle. In response to this growing need, AstraZeneca developed a Patient Opinion Leader (POL) Advisory Panel – a collaborative group of patients and caregivers possessing not only the knowledge of living with
their health condition, but also having expertise in a variety of subjects that intersect nicely with pharma to result in more valuable insights than the average patient could provide.
11:40 Oncology Patient Recruitment: Sponsor Insights
 Archana Sah, Therapeutic Area Leader, Oncology, Genentech, Inc.
Archana Sah, Therapeutic Area Leader, Oncology, Genentech, Inc.
In today’s US landscape in oncology drug development, the competitive marketplace compels sponsors to be more predictive in their enrollment planning. About 80% of clinical trials do not recruit on time and about 30-40%
of sites are low productive sites. This presentation will focus on recruitment planning in oncology studies with recommendations on tactics from landscaping through LPI for a more predictive recruitment planning.
12:05 pm Session Break
 12:10 Bridging Luncheon Presentation: Taking Patient Recruitment into the Next Generation of Clinical Development
12:10 Bridging Luncheon Presentation: Taking Patient Recruitment into the Next Generation of Clinical Development
Kimberly Ray, Vice President, Site and Patient Networks, IQVIA
Using real-world insights to drive better Site identifications. Understanding Site and PI capabilities to drive ideal patients. The importance of communication and advocacy groups to drive engagement and retention.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available
for multiple attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available
for multiple attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Micah Lieberman
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1)
541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute
(CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5456
E: rhandy@healthtech.com