Cambridge Healthtech Institute’s Inaugural
Sensors, Wearables and Digital Biomarkers in Clinical Trials:
Novel Data Sources and Connectivity for Virtual and Remote Trials
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
Clinical research industry is moving toward end-to-end digital clinical trials. The data collection should stay in line with this inevitable change and wearables and point-of-care sensors address this need. Furthermore, digital biomarkers translate new
data sources into clinically actionable insights. The inaugural Sensors, Wearables and Digital Biomarkers in Clinical Trial: Novel Data Sources and Connectivity for Virtual and Remote Trials conference, part of 9th Annual SCOPE Summit, is designed
as a knowledge and experience exchange for clinical data and clinical operations executives. The conference will feature case studies of clinical trials that already employ sensors and wearables as well as discussions of the future steps needed for
digitalization of clinical trials.
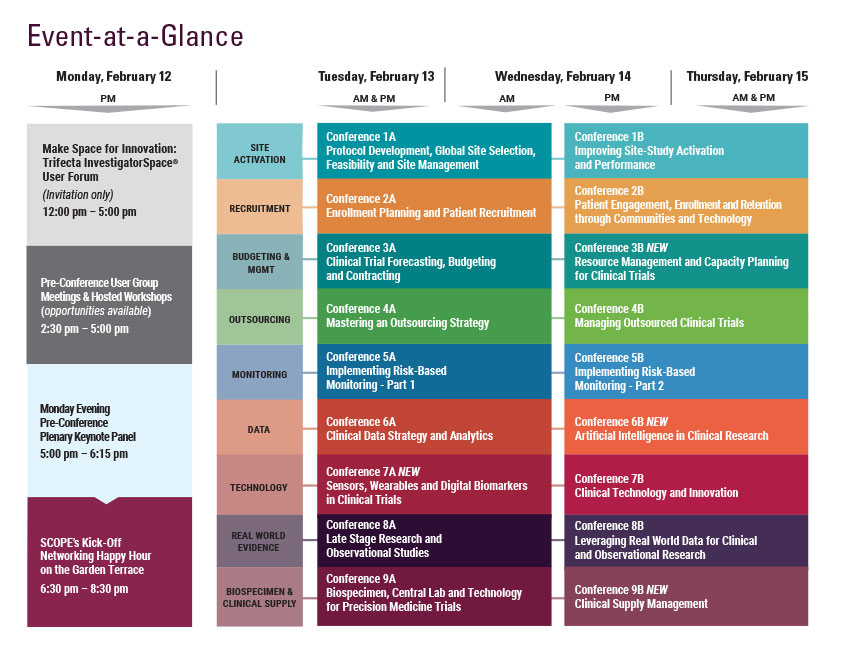
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Christian Gossens, Ph.D., Global Head Early Development Workflows, pRED Informatics, Roche Pharmaceutical Research and Early Development
10:50 Digital Biomarker Development at Roche pRED: How Mobile Technology Can Innovate Clinical Endpoints
 Christian Gossens, Ph.D., Global Head Early Development Workflows, pRED Informatics, Roche Pharmaceutical
Research and Early Development
Christian Gossens, Ph.D., Global Head Early Development Workflows, pRED Informatics, Roche Pharmaceutical
Research and Early Development
Merging the best of two worlds - clinical trials and real world - is now increasingly possible. Mobile sensors are rapidly becoming a part of everybody’s lives. They allow for objective, precise and continuous measurements. We share
our first real world digital biomarker results based on active tests and passive monitoring data - provided by Parkinson’s disease and Multiple Sclerosis patients in clinical trials.
11:10 What Drives the Success (or Failure) in mHealth Platform: Share Learning from GSK PARADE Study
 Michelle Crouthamel, Digital Platform Leader, GSK
Michelle Crouthamel, Digital Platform Leader, GSK
The ability to efficiently develop new medicines for patients with unmet needs is limited by the current model for clinical development. Although the emerging mHealth technologies have the potential to improve the conduct of clinical trials,
the successful implementation requires careful study design and patient engagement. Data and experiences from GSK PARADE study will be summarized, highlighting the learning and opportunities in this new area of clinical development.
11:30 Matching Clinical Needs to Medical Needs
 Ieuan Clay, Ph.D., Group Lead Digital Endpoints, Translational Medicine, Novartis Institutes for Biomedical Research
Ieuan Clay, Ph.D., Group Lead Digital Endpoints, Translational Medicine, Novartis Institutes for Biomedical Research
Due to advances in technology, our ability to capture data in clinical settings is better than ever. How we match the right technology to a medical need, and how we ensure that we are extracting the relevant information from that stream
of data in a robust and sensitive way is the focus of our research. We will present examples and discuss how we are tackling these challenges across different demographics and disease areas.
11:50 Integrating Wearable Sensors in Clinical Trials for Monitoring Real-Life Activities at Home: Developing Clinically-Meaningful Endpoints and Gamification Strategies for
Compliance
 Amir Lahav, Sc.D., Rare Disease Research Unit, Pfizer
Amir Lahav, Sc.D., Rare Disease Research Unit, Pfizer
This talk will provide a visionary approach to developing and validating digital biomarkers using remote health monitoring of daily activities. The core idea of this approach is to create a patient-centric monitoring system that can
objectively quantify meaningful changes in disease progression that would otherwise not be captured by traditional functional assessment in the clinic. This will largely depend on developing data-driven digital tools as well as
engagement and compliance strategies for integrating wearable technology into clinical trials in an efficiently productive fashion.
12:10 pm Clinical Trial Pilots of Wearable Sensors in Diabetes and Asthma
 Martin Karpefors, Ph.D., Informatics Science Director, CardioRenal, Autoimmune & Neuroscience
TA Lead, AstraZeneca
Martin Karpefors, Ph.D., Informatics Science Director, CardioRenal, Autoimmune & Neuroscience
TA Lead, AstraZeneca
Sensors offer a way to transform clinical trial results from snapshot measurements of physiological parameters to continuous remote monitoring, which will enable disease insight, increase patient engagement and control, as well as
give cost savings and operational advantages. However, these promises do not come without challenges. Based on our pilot experiences from (multi-)sensor trials in diabetes and asthma, we present some scientific, analytical and
operational perspectives.
12:30 Session Break
 12:40 Luncheon Presentation: StepWatch™ Accuracy and Reliability Means Greater Probability to Detect Changes in Walking
12:40 Luncheon Presentation: StepWatch™ Accuracy and Reliability Means Greater Probability to Detect Changes in Walking
 Teri Chou, PhD, CEO, Modus Health
Teri Chou, PhD, CEO, Modus Health
Over 100 products exist that measure walking. It is well known that the accuracy of these products varies widely from consumer products such as Fitbit™ to medical devices such as StepWatch™. Independent published studies
of the most used walking monitors emphasize how monitor accuracy and reliability can affect number of study participants needed to detect walking improvements, or alternatively, how power to detect change can be maximized within
a fixed sample size.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Gahan Pandina, Ph.D., Senior Director, Compound Development Team Leader, Neuroscience, Janssen Research & Development
2:05 Novel Biomarkers for Use in ASD Drug Development: State of the Science
 Gahan Pandina, Ph.D., Senior Director, Compound Development Team Leader, Neuroscience, Janssen Research
& Development
Gahan Pandina, Ph.D., Senior Director, Compound Development Team Leader, Neuroscience, Janssen Research
& Development
Autism Spectrum Disorder is a heterogeneous, complex neurodevelopmental disorder affecting 1-2% of the global population. There are currently no medications indicated for the treatment of core symptoms. Recently, research focus has
shifted to the development and potential use of biomarkers to stratify the ASD population, or to assist with measuring treatment outcome change. The presentation will review the current state of the science in development of ASD
biomarkers.
2:30 Key Considerations in Integrating Wearables/Sensors Data in Sponsor’s Ecosystem (AbbVie)
 Aman Thukral, Assistant Director, Data and Statistical Sciences, AbbVie
Aman Thukral, Assistant Director, Data and Statistical Sciences, AbbVie
In pursuit of patient-centric clinical research, sponsors are leveraging wearables and sensors devices in clinical trials. These devices shoot data at a larger volume and velocity compared to traditional systems and require different
skills to collect, integrate, and manage patient data. This session will discuss key considerations in integrating wearables/sensors data in sponsor’s ecosystem.
 2:55
Right-sizing Technology for Early Development Proof of Concept Clinical Trials
2:55
Right-sizing Technology for Early Development Proof of Concept Clinical Trials
 Lorraine Rusch, PhD, President, High Point Clinical Trials Center
Lorraine Rusch, PhD, President, High Point Clinical Trials Center
This discussion focuses on technologies used in early POC studies from a drug development and clinical research facility perspective. 1) Technologies can be utilized to assess endpoints such as glucose, biomarkers and disease progression
in the development of novel therapies for metabolic disorders. 2) Cognitive assessments are crucial tools for the clinical development of CNS-focused therapies. 3) The emerging field of electronic, tablet-based source will be the
future means to maximize data quality and efficiency.
3:20 PANEL DISCUSSION: Digital Technology Adoption and Implementation
Moderator:
 Christian Gossens, Ph.D., Global Head Early Development Workflows,
pRED Informatics, Roche Pharmaceutical Research and Early Development
Christian Gossens, Ph.D., Global Head Early Development Workflows,
pRED Informatics, Roche Pharmaceutical Research and Early Development
Panelists:
 Gahan Pandina, Ph.D., Senior Director, Compound Development Team Leader,
Neuroscience, Janssen Research & Development
Gahan Pandina, Ph.D., Senior Director, Compound Development Team Leader,
Neuroscience, Janssen Research & Development
 Aman Thukral, Assistant Director, Data and Statistical Sciences, AbbVie
Aman Thukral, Assistant Director, Data and Statistical Sciences, AbbVie
 Ieuan Clay, Ph.D., Group Lead Digital Endpoints, Translational Medicine, Novartis Institutes for Biomedical
Research
Ieuan Clay, Ph.D., Group Lead Digital Endpoints, Translational Medicine, Novartis Institutes for Biomedical
Research
 Michelle Crouthamel, Digital Platform Leader, GSK
Michelle Crouthamel, Digital Platform Leader, GSK
 Jaydev Thakkar, Product Innovation Lead, Amgen Digital Health
Jaydev Thakkar, Product Innovation Lead, Amgen Digital Health
This panel will discuss: Wearable sensors’ impact on trials design and execution, Collecting, integrating, and analyzing wearable devices data, and Driving innovation in patient centricity and PROs.
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table
of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group
interrogation and problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can
remove such concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best
practice technology and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Julie Smiley, Director, Product Strategy, Oracle Health Sciences
8:30 Harnessing Digital Technology and Big Data in Clinical Trials and Beyond
Anthony Rowe PhD, Director, R&D IT Business Technology Leader, Immunology TA, Janssen
Measuring physiological and activity-based parameters remotely and continuously via unobtrusive on/off-body sensors or smartphones has the potential to revolutionize our ability to monitor patients between clinic visits and develop
objective markers that track disease trajectory. How can we harness such advances in digital technology and big data analytics to enable more informative and efficient clinical studies and develop patient centric digital solutions
that improve outcomes in the real world?
8:55 Digital Biomarker Implementation, Presentation and Comparability
 Amy Calvin, BS, MT (ASCP), MBA, Digital Strategy and Implementation, Advisor, Eli Lilly and Company
Amy Calvin, BS, MT (ASCP), MBA, Digital Strategy and Implementation, Advisor, Eli Lilly and Company
Over the past few years, data generation is beginning to take a new form. It’s moving from subjective to more objective, from episodic to contemporaneous, and is being generated through connected digital tools that can be
used to quantitatively explain or predict physiological, behavioral, and functional health measures and outcomes. These digital measures are known as digital biomarkers. This presentation utilizes learnings from pilot examples
to examine the implementation, presentation and comparability of digital biomarkers.
9:20 Wearables and Sensors Are Changing the Clinical Trial Process, but What’s the Return on Investment for Making This Dramatic Change in People, Process and Technology?
Deborah Profit, Ph.D., Vice President, Otsuka Information Technology
The advent of wearables and sensors in clinical trials is changing the paradigm of trial designs, clinical teams, outsourcing practices, and ultimately patient engagement. However, what value does sensor/wearable data and these
new trial practices bring, and how quick is the return of investment to the various stakeholders? Otsuka Pharmaceutical Development and Commercialization, Inc. is on the cutting edge of the trial and technology reform,
and will share some critical learnings from “the road less traveled”.
9:45 CO-PRESENTATION: Driving Clinical Strategy & Optimized Design with RWD and Clinical Patient-Level Data
 Qin Ye, MD, Associate Principal, Global RWE Lead, ZS
Qin Ye, MD, Associate Principal, Global RWE Lead, ZS
 Jane Fang, MD, PhD, Head, Biologics, Research & Development Informatics, AstraZeneca/MedImmune
Jane Fang, MD, PhD, Head, Biologics, Research & Development Informatics, AstraZeneca/MedImmune
Historical clinical trial and real-world data hold immense potential in sharpening R&D strategy, optimizing trial design and streamlining operational planning. This data-driven approach has become common practice for
many in the pharmaceutical industry, but there are still hurdles to overcome. For some, the lack of efficient and timeline approach as well as tangible use cases leads to poor adoption and impact. In this presentation
we’ll share how MedImmune leveraged an integrated framework to enable seamless data-driven decision making and process transformation within clinical program planning.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Aman Thukral, Assistant Director, Data and Statistical Sciences, AbbVie
11:15 Identifying, Developing and Incorporating Technology-Derived Endpoints into Clinical Trials: A ‘How-To’ Guide on the Agenda
 Rob DiCicco, Ph.D., Vice President, Clinical Innovation and Digital Platforms,
GSK
Rob DiCicco, Ph.D., Vice President, Clinical Innovation and Digital Platforms,
GSK
Mobile technologies hold enormous promise for clinical research, but uncertainty about how to use the data captured by these devices has slowed progress. This presentation will describe recommendations and tools from
the Clinical Trials Transformation Initiative (CTTI) that aim to change this by providing a pathway for using information gathered from mobile technologies to accelerate the development and evaluation of urgently
needed therapies.
11:40 Making Sense of Sensor Data: A Case Study in Data Quality Evaluation
Bhaskar Dutta, Principal Scientist, Advanced Analytics Center, AstraZeneca
Wearable sensor technology brings the promise of improving management of chronic diseases, identification of drug adverse effects, and use of new efficacy endpoints. Future adoption of wearable sensors in clinical studies
will depend on the usability of the devices and quality of the data. Currently, several sensors are commercially available, hence, requiring a comprehensive review. We carried out a study to compare them in healthy
volunteers and implemented a comprehensive data analysis strategy, which has paved the way for improved designs of future studies involving wearable sensors.
12:05 pm Session Break
12:10 Bridging Luncheon Presentation: Introducing Consent Engineering: The Simplest and Safest Way to Create, Manage and Automate Consent Solutions In-House
 Eric Delente, President, Patient Consent, DrugDev (an IQVIA company)
Eric Delente, President, Patient Consent, DrugDev (an IQVIA company)
The benefits eConsent provides for patient satisfaction and internal efficiencies cannot be overstated, yet the time and expense involved can make it cost-prohibitive for some clinical trials. Consent Engineering is
a new SaaS technology solution enabling sponsors to bring eConsent entirely in-house. Featuring an interface as familiar and intuitive as Microsoft Word, Consent Engineering will completely change the way the industry
does consent. Join us for the world’s first look at this exciting new solution!
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available
for multiple attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available
for multiple attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Marina Filshtinsky, M.D.
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech
Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1)
781.972.5456
E: rhandy@healthtech.com