Cambridge Healthtech Institute’s 8th Annual
Protocol Development, Global Site Selection, Feasibility and Site Management:
Improving Outcomes through Strategy, Relationships, Data and Execution
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
Data-driven global site selection, an optimized protocol development and feasibility assessment process, and effective site management are critical to improving clinical trial timelines and outcomes. Too often companies fail to learn from past mistakes
and take the same approach to trial planning and execution. In order to overcome challenges in clinical trial planning, operations and site management leaders should learn from the best practices of their peers, utilize data and technology to support
decision making, and improve communication and relationships between Sites, CROs, and Sponsors. CHI’s 8th Annual Protocol Development, Global Site Selection, Feasibility and Site Management will cover the
topics one should consider when planning and implementing a trial.
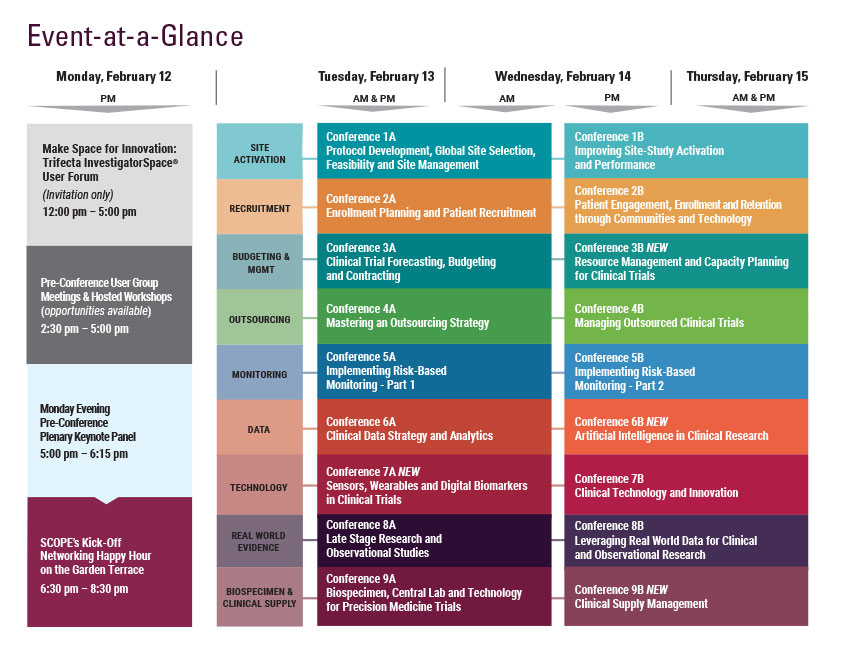
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Christopher Conklin, Director, Feasibility Center of Excellence, Pfizer
10:50 Trends in Protocol Design Practice and Its Impact on Performance
 Stella Stergiopoulos, Project Manager, Tufts Center for the Study of Drug Development,
Tufts University
Stella Stergiopoulos, Project Manager, Tufts Center for the Study of Drug Development,
Tufts University
Co-developed with Ken Getz, MBA, Director, Sponsored Research Programs, Tufts CSDD; Chairman, CISCRP
The scientific and operating demands of protocol designs have increased rapidly during the past decade due to numerous factors. This talk characterizes trends in, and the forces driving, protocol design practices. The impact of these practices
on clinical trial performance and economics will be discussed.
11:15 Maximizing Site Contributions in a Competitive Landscape
 Kelly White, Director, Global Operations Oncology, Global Trial Optimization, Merck & Co.
Kelly White, Director, Global Operations Oncology, Global Trial Optimization, Merck & Co.
Sites submit trial proposals for participation and are selected (or not selected) without much insight into the protocol or rationale for why they have been allocated a particular number of patients. Sponsors create competitive recruitment strategies
that don’t allow sites to plan or manage their resources, so even when sites have the ability to enroll more patients, they often don’t have the resources. I will set forth some proposals for how we might “stop the insanity”
of peppering the world with sites and focus on a collaborative model where sites and sponsors plan their recruitment and resources synergistically with the goal of making both of them successful.
11:40 Feasibility... Decisions, Decisions, Decisions: Early Planning and Internal-External Models
 John Oidtman, Senior Vice President, Head of Global Clinical Operations, EMD Serono
John Oidtman, Senior Vice President, Head of Global Clinical Operations, EMD Serono
This presentation will focus on the steps that sponsors must consider when planning clinical trial conduct. It will provide insights into approaches to optimize the early planning process and will also provide perspectives on the pros and cons
of internal vs. external business models.
12:05 pm CO-PRESENTATION: Strengths and Limitations of Data in Clinical Trial Feasibility
 Martin Lee, MD, Vice President, Scientific Services, Scientific Affairs, PRA Health Sciences
Martin Lee, MD, Vice President, Scientific Services, Scientific Affairs, PRA Health Sciences
 Jacqui Whiteway, PhD, Senior Director, Feasibility, Scientific Affairs, PRA Health Sciences
Jacqui Whiteway, PhD, Senior Director, Feasibility, Scientific Affairs, PRA Health Sciences
There is an increasing emphasis on the use of data in clinical trial planning, especially in projecting enrollment rates and in selecting countries and sites for participation. Our presenters will identify the strengths, limitations and optimal
use of existing and emerging data sources; discuss the importance of direct site interactions in the planning process; and recommend a systematic approach to optimal clinical trial planning.
12:30 Session Break
12:40 Luncheon Presentation: Combining Patient Registries with Investigator Site Experience to Enhance Trial Design & Clinical Trial Performance
 Dawie Wessels, PhD, CMO, Synexus Clinical Research
Dawie Wessels, PhD, CMO, Synexus Clinical Research
Alzheimers Disease studies are complex, long and very expensive. An estimated 50,000 patients are required in the next few years and the complexity of the patients are increasing, making recruitment more challenging. The talk will cover an approach
using registries in combination with biomarkers (genotyping, memory testing) as a promising way to address this problem.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Kelly White, Director, Global Operations Oncology, Global Trial Optimization, Merck & Co.
2:05 Accelerating Drug Development through Protocol Harmonization: TransCelerate’s Common Protocol Template
 Robert DiCicco, Pharm.D., Vice President, Clinical Innovation and Digital Platforms, GlaxoSmithKline
Robert DiCicco, Pharm.D., Vice President, Clinical Innovation and Digital Platforms, GlaxoSmithKline
Increasing complexity in protocols makes implementation and reporting difficult and the lack of consistency compounds the issue. A significant opportunity exists for an improvement in quality and simplification through protocol harmonization,
as all protocols rely on the same health care and regulatory infrastructure for design, review and implementation. This session will explore the collaboration between TransCelerate, FDA, and NIH to achieve alignment on a common protocol structure.
It will also describe how TransCelerate’s CPT enables use of clinical data standards, as well as next steps towards automation and data traceability from protocol through to downstream processes.
2:30 CASE STUDY CO-PRESENTATION: How the Business Embedded the Tech-Enabled CPT in Our Business Process and Extended It to Extract Data for Downstream Processes
 Bina Rathod, Associate Director & Business Lead, MRL IT Global Clinical Development, Merck & Co.
Bina Rathod, Associate Director & Business Lead, MRL IT Global Clinical Development, Merck & Co.
 Mitzi Allred, Director, Clinical Research, Clinical Content Standards, Merck & Co.
Mitzi Allred, Director, Clinical Research, Clinical Content Standards, Merck & Co.
This presentation will cover how our business partners leverage the tech enabled CPT to make protocol data and metadata available to downstream clinical processes, tools and applications to optimize reusability with minimum manual intervention.
We will cover from protocol creation and downstream process to clinical study report finalization.
 2:55 New Data-Driven
Methods to Score Protocols for Patient Burden and Optimize Trial Design
2:55 New Data-Driven
Methods to Score Protocols for Patient Burden and Optimize Trial Design
 Denise Messer, Scientific Advisor, Evidence Driven Design, IQVIA
Denise Messer, Scientific Advisor, Evidence Driven Design, IQVIA
This presentation explores a method to proactively capture data on perception of clinical trial burden via survey from a population of patients and translate into an algorithm to quantify and score patient burden for a trial and for each scheduled
visit. During the design phase, this burden assessment can be used to show the impact of changes on the burden level of the trial, compare relative burden of competitor trials, and highlight recruitment and retention risks to drive operational
mitigation and optimize trial success.
3:20 PANEL DISCUSSION: Current and Emerging Protocol Optimization Design Strategies
 Christopher Conklin, Director, Feasibility Center of Excellence,
Pfizer
Christopher Conklin, Director, Feasibility Center of Excellence,
Pfizer
 Robert DiCicco, Pharm.D., Vice President, Clinical Innovation and Digital Platforms, GlaxoSmithKline
Robert DiCicco, Pharm.D., Vice President, Clinical Innovation and Digital Platforms, GlaxoSmithKline
 John Oidtman, Senior Vice President, Head of Global Clinical Operations, EMD Serono
John Oidtman, Senior Vice President, Head of Global Clinical Operations, EMD Serono
A number of protocol design optimization strategies have been introduced and implemented including protocol authoring tools, feasibility review mechanisms, patient advisory boards, and pre-planning simulations. This talk touches on optimization
strategies and provides data on their impact to date where available. Several protocol design optimization strategies will also be discussed including the adoption and use of adaptive designs and patient centric approaches. This introductory
talk will be followed by a panel discussion among pharmaceutical and biotechnology company professionals. Panel members will discuss:
- Optimization strategies that most resonate with their respective organizations and why
- Experience with various strategies and lessons learned
- Adoption challenges and impact following implementation
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest
and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and
problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Day 1 | Day 2 | Download Brochure
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove such
concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice technology
and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Michael Bonavilla, Senior Sales Executive, Bio-Optronics
8:30 An Integrated Approach to Feasibility: Driving Operational Strategy and Planning Using Historical Data and Local Experts
 Kate Boneck, Associate Director, Global Trial Optimization, Merck & Co.
Kate Boneck, Associate Director, Global Trial Optimization, Merck & Co.
Historical benchmarking, real time expert input, and data-driven global site selection are critical to improving clinical trial timelines and outcomes. This presentation will share an approach to the feasibility process that uses both
data assets as well as expert input to support clinical trial teams in optimizing trial planning and execution.
8:55 Assessing New Data-Driven Approaches Informing Global Investigative Site Selection
 Stella Stergiopoulos, Project Manager, Tufts Center for the Study of Drug Development,
Tufts University
Stella Stergiopoulos, Project Manager, Tufts Center for the Study of Drug Development,
Tufts University
A 2016 Tufts CSDD study has demonstrated that current shifts in the investigative site landscape will increase the need for data-driven approaches to inform global investigative site selection, as the landscape remains highly fragmented,
nascent, and unstable. In light of these findings, Tufts CSDD conducted two studies assessing current industry trends and strategies on end-to-end site identification through study start-up as well as assessing the types of data that
can be gathered from BMIS and clinicaltrials.gov. Industry strategies around data-driven solutions will be discussed.
9:20 CASE STUDY: Does Use of Public Data at the Study Level Result in a Different Planning Strategy When Compared to Shared Site-Level Data?
 Christopher Conklin, Director, Feasibility Center of Excellence, Pfizer
Christopher Conklin, Director, Feasibility Center of Excellence, Pfizer
This case study will compare use of shared CTMS data at the site-level vs. publicly available data at the study level in support of study planning for a Phase III study of 1000 subjects with moderate to severe plaque psoriasis. The analysis
focuses on two planning endpoints: number of sites required and expected number of patients enrolled per site. Publicly available data underestimates the number of sites required by 21%, and overestimates the enrolment rate by 53%.
The session will conclude with a discussion of the potential impact on the need for rescue studies, projected trial enrolment duration, country/site selection, and the benefits of cross-company sharing of CTMS.
 9:45 Translating Registry & Health Informatics Data to Site Performance: Opportunities to Accelerate Feasibility & Enrollment
9:45 Translating Registry & Health Informatics Data to Site Performance: Opportunities to Accelerate Feasibility & Enrollment
 Earl Seltzer, Director, Global Feasibility, Syneos Health
Earl Seltzer, Director, Global Feasibility, Syneos Health
In the age of burgeoning data sources and data driven clinical trial planning, many questions remain about how these data, particularly at the patient level, are actually translated to a viable trial delivery solution. This session will
cover the use of patient data to facilitate trial feasibility and site selection from a CRO perspective, including lessons learned, current gaps and progress to address them globally, with a focus on understudied indications and rare
diseases.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Michael Bonavilla, Senior Sales Executive, Bio-Optronics
11:15 CO-PRESENTATION: Rolling Feasibility in Competitive Immuno-Oncology Trials
 Jane Fang, M.D., Head, R&D Information, Clinical Biologics, MedImmune/AstraZeneca
Jane Fang, M.D., Head, R&D Information, Clinical Biologics, MedImmune/AstraZeneca
 Jill Loftiss, Head, Clinical Operations, Oncology, MedImmune/AstraZeneca
Jill Loftiss, Head, Clinical Operations, Oncology, MedImmune/AstraZeneca
With the rapid development of cancer immunotherapy drug development, competition in the immuno-oncology (IO) trials is fierce. The precision medicine behind trial design is driving rapid change in the way of traditional feasibility assessment.
As a result, the innovative approach of Rolling Precision Feasibility has been developed to provide ongoing comprehensive analyses covering trial competitive landscape, patient population, disease epidemiology, precision recruitment
rate, regulatory approval landscape, country and site selection strategy, impact of standard of care change, etc.
11:40 PANEL DISCUSSION: Evidence for Success: Which Data Sources and Data Elements to Use in Study Planning and Site Selection
Moderator:
 Elisa Cascade, President, Data Solutions, DrugDev
Elisa Cascade, President, Data Solutions, DrugDev
Panelists:
 Julie Argento, Principal Data Scientist, Center for Design and Analysis, Amgen
Julie Argento, Principal Data Scientist, Center for Design and Analysis, Amgen
 Shawn Tedman, MBA, Head, Product Offerings, Clinical Trial Optimization Solutions (CTOS), QuintilesIMS
Shawn Tedman, MBA, Head, Product Offerings, Clinical Trial Optimization Solutions (CTOS), QuintilesIMS
When it comes to data to plan clinical trials, drive country selection, predict enrollment, and find sites, more may be better, but each data source often adds cost, time, and complexity to the process. While the published literature cites
4 factors for predicting successful site performance: investigative site focus, experience, past performance, time to randomize first subject, actual best practice doesn’t necessarily match the theory published in the literature.
In this session, industry experts will review in detail which specific data sources and data elements they use to support evidence-based planning, and the relative importance and sequence in which these sources/elements are used.
- Understand data sources and elements available to support study planning and site selection
- Identify strategies for maximizing return while minimizing cost and burden
- Gain insight into which data sources/elements are most powerful in an evidence-based approach
12:05 pm Session Break
12:10 Bridging Luncheon Presentation: Approaches to Evidence Based Site Planning in Trial Design
 Gavin Coney, Head, Clinical, Clarivate Analytics
Gavin Coney, Head, Clinical, Clarivate Analytics
We will explore approaches to ensuring that your study planning is based on the broadest evidence base. We will demonstrate how additional manually curated intelligence can complement existing data sources to identify relevant
insights based on similar studies and provide specific insights into critical trial design and planning decisions.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Micah Lieberman
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E:
mlieberman@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com