Cambridge Healthtech Institute’s 2nd Annual
Managing Precision Medicine Trials:
Biomarker and Genomics Considerations
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
The concept of personalized or precision medicine, with medical decisions, practices, and/or products being tailored to the individual patient, has brought to life several types of clinical trials that involve biomarkers. Effective management of these trials can be complicated and requires specific operational approaches. CHI’s Second Annual "Managing Precision Medicine Trials" symposium is designed as an educational event to discuss solutions to overcome operational and scientific challenges with various types of studies including trials with biomarker-based stratified trials, trials for biomarker discovery and trials with biomarkers as end points. Study design specifics and operational challenges in biomarker-involved clinical trials will be discussed by experts from top pharmaceutical companies.
Final Agenda
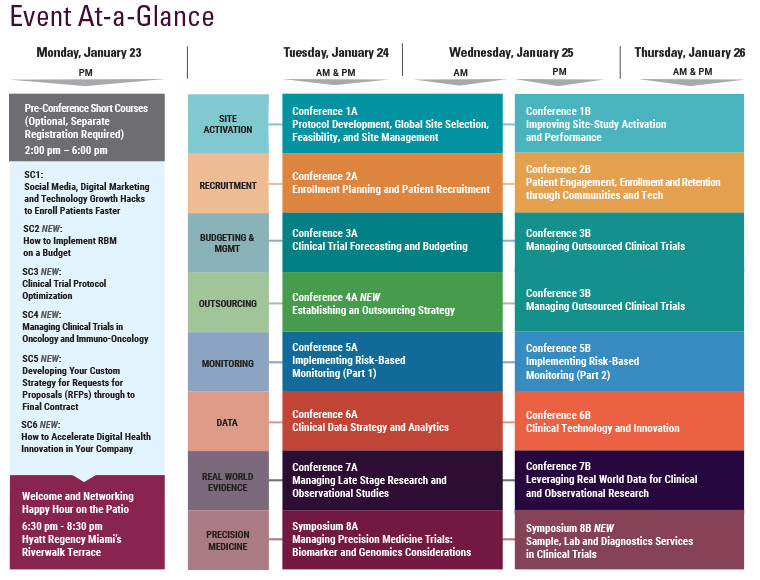
Monday, January 23
1:00 pm Short Course Registration
Recommended Short Course*
2:00 – 6:00 pm Managing Trials in Oncology and Immuno-Oncology
* Separate registration required
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Brenda Yanak, Ph.D., Director, Precision Medicine Leader, Clinical Innovation, Pfizer
10:50 FEATURED PRESENTATION: Operational Challenges and Opportunities in Design and Implementation of Biomarker Selective Clinical Trials
 Bardia Akbari, Pharm.D., Vice President, Product Global Development, Oncology Genentech, Inc.
Bardia Akbari, Pharm.D., Vice President, Product Global Development, Oncology Genentech, Inc.
Advancements in diagnostic technologies and greater understanding of the underlying molecular pathophysiology of diseases has led to propagation of discovery and development of targeted therapies. Despite the potential to clear higher efficacy bar, early and late stage development of target therapies in biomarker-selective patient populations introduces unique scientific, operational, regulatory, and commercial challenges. In this talk we will examine some of these challenges.
11:30 Conducting Genomic Research in Global Clinical Trials
 Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist, Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research
Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist, Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research
This presentation will provide an overview of Merck’s clinical pharmacogenomics strategy. Challenges encountered in conducting genomic research in the context of global clinical trials will be discussed as well as strategies and solutions to manage requirements from global health authorities and IRB/IECs
12:10 pm Interactive Discussion: Clinical Operations to Adjust to the Concept of Personalized Medicine
Bardia Akbari, Pharm.D., Vice President, Product Global Development, Oncology Genentech, Inc.
Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist, Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research
Topics to be discussed include but are not limited to the following:
- Applying the concept of personalized/precision medicine to clinical development
- Unique operational challenges of early and late stage development of therapies in biomarker-selective patient population
- Leveraging pharmacogenomics in clinical research
- Operationalizing biomarker-based randomization
- Multi-drug multi-sponsor trials: New paradigm leads to new challenges
- Collaboration and exchange of information regarding molecular profiling and treatment selection
- Regulatory challenges and impact on FDA submission strategies
- Commercial challenges and solutions
12:40 Enjoy Lunch on Your Own
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Karina Bienfait, Ph.D., Head, Global Genomics Policy, Process and Compliance, Principal Scientist, Clinical Pharmacogenomics and Operations, Genetics and Pharmacogenomics (GpGx), Translational Medicine, Merck Research
2:05 Operationalizing Precision Medicine
 Brenda Yanak, Ph.D., Director, Precision Medicine Leader, Clinical Innovation, Pfizer
Brenda Yanak, Ph.D., Director, Precision Medicine Leader, Clinical Innovation, Pfizer
Success stories in Precision Medicine generally focus on an individual scientific scenario, for example, in the Oncology space. Other success stories focus on a specific technology implemented. This talk will focus on building policy and process infrastructure to operationalize Precision medicine at the enterprise level, as well as discuss the changing external environment and how that impacts infrastructure considerations.
2:50 Clinical Trials in Oncology
Jill Loftiss, Senior Director, Oncology Clinical Operations, Astra Zeneca/MedImmune
3:45 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Download Brochure
Wednesday, January 25
7:15 am Registration
7:45 Breakfast Presentation (Sponsorship Opportunity Available) or Morning Coffee
8:25 Chairperson’s Remarks
Amir Handzel, Ph.D., Statistical Science Director, Astrazeneca
8:30 Critical Decisions in Designing and Implementing Precision Medicine Trials
 Jim Stolzenbach, President, Jim Stolzenbach Consulting, LLC (former Vice President for Abbvie Renal and Immunology Therapeutic Development)
Jim Stolzenbach, President, Jim Stolzenbach Consulting, LLC (former Vice President for Abbvie Renal and Immunology Therapeutic Development)
This presentation will describe the experience and considerations in designing and implementing personalized/precision medicine trials. Protocol development, company-wide collaboration strategies and trial oversight will be discussed
- What is the state of the science that will support the design elements of the trial?
- Is the trial designed to “Learn” or “Confirm”?
- Is there organizational alignment on each function’s role in conducting the trial?
9:00 Use of Biomarkers to Guide Clinical Development in Inflammatory Disease
Mary Flack, Senior Clinical Program Leader, Boehringer-Ingelheim
Sudha Visvanathan, Translational Medicine, Boehringer-Ingelheim
Patients with inflammatory diseases have disordered immune responses. In the case of psoriasis, this leads to the skin plaques that are the hallmark of the disease. It is increasingly recognized that there is also systemic immune dysregulation associated with an increased risk of metabolic syndrome and the potential for accelerated atherosclerosis. Utilizing comprehensive biomarker analysis integrated within the Phase I and II clinical studies, Boehringer Ingelheim has identified molecular biomarkers in the skin associated with positive clinical responses. In addition, we are using methods to identify pro-inflammatory adipokines in the blood that may influence the increased risk of cardiovascular disease and may be modulated by treatment. Through integration of clinical, immunohistochemical and RNA sequencing data, we have begun to identify patterns that can differentiate blockade of one therapy against another.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Mary Flack, Senior Clinical Program Leader, Boehringer-Ingelheim
11:15 The VA Research Precision Oncology Program
 Louis Fiore, M.D., Executive Director, Department of Veterans Affaris, MAVERIC
Louis Fiore, M.D., Executive Director, Department of Veterans Affaris, MAVERIC
The Department of Veterans has created a national consortium of medical centers that participate in precision oncology clinical trials. The intention of the program is to allow all patients equal access to clinical trials, despite geographical variation relative to cancer centers. Different models of patient participation are in place and under development to enable recruitment of patients from medical centers with a wide range of expertise. The Program is participating in studies sponsored by the National Cancer Institute, American Society of Clinical Oncology and a variety of pharmaceutical companies.
11:40 Innovative Trial Designs for Precision Medicine: Intertwining Science and Clinical Operations
 Amir Handzel, Ph.D., Statistical Science Director, Astrazeneca
Amir Handzel, Ph.D., Statistical Science Director, Astrazeneca
A decade ago the iSPY and BATTLE trials ushered in a new type of clinical trials specifically tailored for Precision Medicine. These novel designs addressed difficulties in using standard randomized trials for testing increasingly complex scientific hypotheses and operational obstacles. The new Umbrella and Basket trial designs have solved some previous challenges but raised new ones requiring tight cooperation between scientific, clinical development and clinical operations teams.
12:10 pm Enjoy Lunch on Your Own
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com