Cambridge Healthtech Institute’s 9th Annual
Clinical Data Strategy and Analytics:
Enabling Data Driven Clinical Trials
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
E-clinical technology is changing the landscape of the clinical research industry and healthcare IT in general. Digitalization of healthcare data, mobile data capture technologies, and cloud storage of data are a few of the main technological advances that influence clinical research informatics. The technological advances have been coupled with novel data visualization solutions, and this powerful duo is helping to develop a new paradigm of data-driven clinical trials. Cambridge Healthtech Institute’s Ninth Annual "Clinical Data Strategy and Analytics" conference is designed to bring together clinical research informatics experts to discuss the challenges and find solutions necessary to navigate and thrive in this rapidly changing environment.
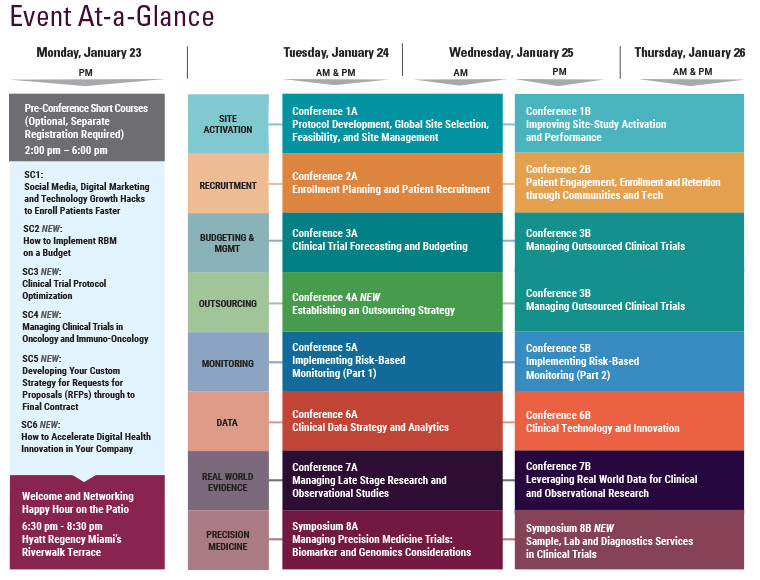
Final Agenda
Monday, January 23
1:00 pm Short Course Registration
Recommended Short Course*
2:00 – 6:00 pm How to Implement RBM on a Budget
* Separate registration required
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Balazs Flink, M.D., Clinical Trial Analytics Lead, R&D Business Insights and Analytics, Bristol-Myers Squibb
10:50 Quality, Risk, Analytics, and Speed: Industry Trends and the Impact on the Direction of Clinical Data Management
 Gary Thompson, Senior Director, Data Sciences and Solutions and Biometrics Sourcing, Eli Lilly
Gary Thompson, Senior Director, Data Sciences and Solutions and Biometrics Sourcing, Eli Lilly
The clinical development industry is experiencing a confluence of several streams of change - including rapid adoption of risk-based quality management approaches, increasing receptivity to electronic data collection, and application of advanced analytics and data visualization to clinical trial execution and analysis. Each of these changes would be significant by itself, but together they present unprecedented challenges and opportunity. This presentation explores the implications of these trends and how clinical data strategy will increasingly become the key to successful clinical development.
11:15 Analytics Linked to Strategy & Execution
 Balazs Flink, M.D., Clinical Trial Analytics Lead, R&D Business Insights and Analytics, Bristol-Myers Squibb
Balazs Flink, M.D., Clinical Trial Analytics Lead, R&D Business Insights and Analytics, Bristol-Myers Squibb
BMS decided to integrate all corporate analytics functions under one organization to drive enterprise level decision-making through data and organically improve the way these matrixed teams work. The result is integrated, predictive analytics that help drive R&D strategy and execution, with clear ties to long-term financial impacts. This presentation highlights the concept and the early results including the challenges and cultural aspects of the change.
11:40 Analytics to Drive Better Decisions in Clinical Development
Matt Austin, Director of Data & Analytics, Amgen
Teams involved in the clinical development process can use analytics to help make better decisions. This talk will include examples of predictive models for enrollment, optimization of the geographic distribution of sites and Power vs. assurance.
12:05 pm How One Mid-Sized CRO Turned Sponsor Variability into An Opportunity to Innovate
 Brion Regan, Product Manager, ERT Insights Cloud, ERT
Brion Regan, Product Manager, ERT Insights Cloud, ERT
Contract Research Organizations (CRO) are ground zero for both the technical challenges and innovation opportunities our industry faces today. Two key challenges these organizations face is process and system variability across customers (different systems, monitoring report templates, oversight requirements, etc.). This presentation will explore how one mid-sized CRO turned these challenges into an opportunity for innovation, and combined data analytics with modern CTMS capabilities to simplify complex processes, automate information sharing, and optimize sponsor collaboration.
12:40 Luncheon Presentation: Navigating Clinical Data Technology Outsourcing
 John Fontenault, Senior Vice President, Operations, OmniComm Systems, Inc.
John Fontenault, Senior Vice President, Operations, OmniComm Systems, Inc.
From large pharma to small biotech, clinical data technologies are critical to your ability to deliver clinical data on time with high quality. Managing these technologies across potentially multiple CRO and vendor relationships can be challenging as processes and reported KPIs may vary. This presentation will focus on communication techniques and key analytics to monitor the health and productivity of your vendor relationships.
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Matt Austin, Director of Data & Analytics, Amgen
2:05 Centralized Monitoring in Action: A Case Example
 John Rodermund, Global Head, Clinical Research Data Management and Centralized Monitoring, AstraZeneca
John Rodermund, Global Head, Clinical Research Data Management and Centralized Monitoring, AstraZeneca
Centralized Monitoring in the world of Risk-Based Monitoring can be taken as two different approaches (Quality-Based or Efficiency-Based). The talk will show an example of a Quality-Based approach to Centralized Monitoring.
2:35 Risk-Based Monitoring in a Multi-Study International Phase III Program
 Mary Flack, M.D., Senior Clinical Program Leader, Immunology, Boehringer-Ingelheim
Mary Flack, M.D., Senior Clinical Program Leader, Immunology, Boehringer-Ingelheim
Stuart Shaw, Biostatistics & Data Monitoring, Boehringer-Ingelheim
A Phase 3 program is being conducted encompassing 4 major studies, involving 280 sites in 17 countries. These sites enrolled over 2100 subjects in 6 months, so site monitoring needed to keep pace with the rapid enrollment. Based on recommendations from Transcelerate, Boehringer-Ingelheim (BI) has developed a tool to identify study risks and evaluate the risk level of each trial. This tool has been used for risk assessment in several previous studies, but this was the first time it was used at a program level. Based on the risks identified, we produced weekly reports (SCORE) rating site performance in 6 different areas (Safety, Investigational Product, Recruitment and Discontinuation, Data Quality, Staffing facilities & supplies, and Program Specific parameters). These reports also included aggregate scores for sites across studies, across countries and for the entire program. Scores were color coded (red, yellow, green) for rapid visual assessment of sites at risk. Direct data capture was used wherever possible to facilitate real time assessment of site performance. While most sites remained low risk, several moderate risk and a few high risk sites were identified quickly so that issues could be resolved in a timely manner.
 3:10 Simplifying Visit Reporting
3:10 Simplifying Visit Reporting
 Duncan Scattergood, President, OnTheMove Software
Duncan Scattergood, President, OnTheMove Software
Organizations now realize that providing CRAs with IT systems that simplify rather than hinder has the potential to simultaneously improve employee satisfaction and effectiveness. This presentation looks at a case study with over 800 CRAs to see how one organization delivered a significantly improved CRA experience using OnTheMove Clinical.
 3:30 Clinical Data: Legacy Burden or Strategic Asset
3:30 Clinical Data: Legacy Burden or Strategic Asset
 Eliot Knudsen, Lead, Data Science, Tamr
Eliot Knudsen, Lead, Data Science, Tamr
Are you using your clinical data to its full potential? Despite the importance of R&D analytics, groups struggle to integrate data. Learn the best practices of preparing data for clinical analytics, as well as how to combine machine learning with subject-matter expertise to accelerate and scale the data integration process.
3:50 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, January 25
7:15 am Registration
7:30 Breakfast Presentation: eConsent: Put "Informed" Back in Informed Patient Consent
 Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Electronic informed consent makes the consenting process more efficient and effective for staff, sponsors, monitors, and most importantly patients by presenting the information in formats and language in which patients are comfortable. Join us for breakfast to learn the best practices, latest technological advances, and proven benefits of deploying an eConsent solution - including the impact it has on improving patient satisfaction and retention and help us put "informed" back in patient consent process.
8:25 Chairperson’s Remarks
Pam Duffy, IT Lead, Core Clinical Solutions & Services, Pfizer
8:30 Integrating In-House and Cloud-Based Infrastructure
 Pam Duffy, IT Lead, Core Clinical Solutions & Services, Pfizer
Pam Duffy, IT Lead, Core Clinical Solutions & Services, Pfizer
Cloud computing brings numerous advances such as speed, agility, flexibility, elasticity and innovation. What does that mean to clinical development and how can we take advantage of it? This presentation will share Pfizer’s experience with implementing cloud computing in clinical trials and elaborate on the problems and solutions for integrating in-house and cloud-based infrastructure.
8:55 Leverage Strategic CRO Partnership in Clinical Innovations
Heather Zigmund, Pharm.D., Senior Director and Head of Clinical Services, MedImmune
Formal partnership governance and oversight plays an important role to play in improving partnership effectiveness and efficiency. However, getting the most out of a partnership is not just about standardising and streamlining existing processes. It’s about continually embracing the entrepreneurial spirit to work smarter. This presentation will review how to leverage strategic CRO partnerships to advance clinical innovations within your organization.
9:20 Data Visualization Techniques for Safety Signal Detection and Efficacy Evaluation in Clinical Studies
 Mukta Tripathi, Medical Data Review Specialist, PDC Business Ops, Product Development, Genentech
Mukta Tripathi, Medical Data Review Specialist, PDC Business Ops, Product Development, Genentech
At Genentech/Roche data visualization approach to clinical study data has revolutionized the process of individual data review as well as aggregate review to identify outliers or detection of safety signal. This talk focuses on advantages of implementing data visualization tool for efficiency and scenario building to study trends within clinical data.
 9:45 Using Analytics to Drive a New-Way-of-Working
9:45 Using Analytics to Drive a New-Way-of-Working
 Lawrence Florin, Clinical Leader, Life Sciences Research & Development, Cognizant
Lawrence Florin, Clinical Leader, Life Sciences Research & Development, Cognizant
Aggregating information from multiple sources provides researchers insights to open new lines of inquiry, to avoid and/or mitigate risks and to drive actions. Synthesizing large data volumes to make sensible decisions requires a change in approach that encourages reliance on simpler, intuitive data visualizations while still permitting more granular data exploration, when needed. This presentation will discuss Cognizant collaboration with biopharma companies to develop technology and change management tools to bring about this result.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Thomas Haag, Learning and Process Lead, Digital Development, Novartis Pharmaceuticals
11:15 eSource & Data Integrity
 Thomas Haag, Learning and Process Lead, Digital Development, Novartis Pharmaceuticals
Thomas Haag, Learning and Process Lead, Digital Development, Novartis Pharmaceuticals
When faced with the concept of adopting eSource in your organizations clinical trial program, what are the key domain changes faced related to the data collection process? This presentation will examine the Domains of Changes related to the Adoption of eSource faced by Sponsors, Suppliers, and Health Authorities. Key changes in the areas of Data, Application, and Technology along with the less obvious changes to Process, Organization and Location will be addressed.
11:40 TransCelerate Clinical Data Transparency: Returning Plain Language Summaries Implementation Guide and Toolkit
Jaime Houde, Manager, Clinical Trial Transparency, EMD Serono, Inc.
New regulatory requirements and increasing patient and consumer expectations have brought the development and distribution of aggregate results summaries in plain language to the forefront as a new arena for clinical trial transparency. Sponsors will need to establish new policies and procedures for effective and efficient delivery with awareness of the challenges and potential risks and ways to mitigate those risks. The TransCelerate Data Transparency workstream has developed an Implementation Guide and Toolkit to help sponsors think through all aspects of the process and has provided examples of best practices in the toolkit.
 12:05 pm Bridging Luncheon Co-Presentation: Bridging the Clinical Data Structure Gap for Holistic RBM: How Fully Integrated Data Empowers Risk Management
12:05 pm Bridging Luncheon Co-Presentation: Bridging the Clinical Data Structure Gap for Holistic RBM: How Fully Integrated Data Empowers Risk Management
 Sudeep Pattnaik, MS, MBA, Co-Founder & CEO, ThoughtSphere, Inc.
Sudeep Pattnaik, MS, MBA, Co-Founder & CEO, ThoughtSphere, Inc.
 Pankaj Manon, Co-Founder & CTO, ThoughtSphere, Inc.
Pankaj Manon, Co-Founder & CTO, ThoughtSphere, Inc.
Learn how a purpose-built for clinical trials data lake and informatics solution lets you take advantage of leading-edge big data practices for holistic RBM, removing the data structure barrier. Data integration brings to life risks that are not apparent when you examine the data from one source alone. Now accessing all clinical data regardless of its format in near real-time is possible, available in one place enabling actionable insights.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
Download Brochure
Stay on and attend Part 2: Clinical Technology and Innovation
Keynotes | Monday Short Courses | Speaker Biographies
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com