Cambridge Healthtech Institute’s 3rd Annual
Managing Outsourced Clinical Trials
Measuring Performance & Forming Quality Partnerships
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
As more clinical trial activities are outsourced to contract research organizations (CROs) and other third party vendors, sponsors and their partners must learn to form effective and quality partnerships. Effective management of outsourced clinical trials requires realistic and explicit expectations from each partner in the outsourcing relationship as well as the ability to measure partnership and project performance and quality. Cambridge Healthtech Institute’s “Managing Outsourced Clinical Trials” conference features case studies and lessons learned from sponsors and CROs on how to measure vendor quality and performance, as well as optimize the outsourcing partnership to achieve more efficient clinical trials.
Final Agenda
Wednesday, January 25
12:05 pm Bridging Luncheon Presentation: Introducing DrugDev Spark™ - Technology to Transform Clinical Operations
 Brett Kleger, Chief Commercial Officer, DrugDev
Brett Kleger, Chief Commercial Officer, DrugDev
DrugDev Spark™ is revolutionary clinical technology that brings all administrative solutions sponsors, CROs and sites need to run a trial together into one unified solution suite with a single sign on. Featuring solutions from site selection and activation, to payments, training and eConsent, all tied together with the DrugDev Golden Number DrugDev Spark is primed to transform the way global clinical trials are run. Join us for lunch and an exclusive preview at SCOPE!
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairperson’s Remarks
Christopher Rull, Vice President, Head of Business Development & Account Management, UBC
4:05 Sourcing Need to Provider Selection: Part II: A Strategy for Translating Contracts into an Effective Partnership
Marija Nikolic, Associate Director, Development Operations, Contracts & Outsourcing, Vendor Management, Astellas Pharma Global Development, Inc.
You’ve navigated the stormy sea of provider evaluation and selection and the contract is signed. What next? How do you carry the momentum forward into the post contract phase to evolve the relationship from sponsor-provider to sponsor-partner? Which providers should be considered for true partnership? What makes a partnership successful? This talk will provide proposals for how to make the evolution from provider to partner. Suggestions for what a good partnership can look like and which provider categories are most likely to benefit from a true partnership.
4:30 How a Clinical Team Avoided $420k in CRO Change Orders
 Rick Morrison, CEO, Comprehend
Rick Morrison, CEO, Comprehend
Learn how a leading clinical development team revolutionized their relationships with their CROs. Understand the best practices they put in place to continuously manage study quality and achieve milestones on-time and on-budget across their portfolio of trials. This session will outline the 5 best practice steps they took to avoid delays and costly overruns in areas such as: enrollment, site productivity, medical monitoring, and data management.
4:55 Co-Presentation: Exploring the Linkages of End-to-End Business Process with Operational Performance for Successful Partnerships in Clinical Development
 Charlotte French, Senior Director, Global Head, Contracting & Outsourcing, EMD Serono
Charlotte French, Senior Director, Global Head, Contracting & Outsourcing, EMD Serono
Christopher Rull, Vice President, Head of Business Development & Account Management, UBC
This session will provide the participants with an overview of the End-to-End Business Processes required to support the successful Operational Delivery of clinical trials. In today’s economic and competitive climate there is an increased need for service providers to support their Partners with the necessary financial transparency throughout a project lifecycle, from initial budgeting to provision of forecasting data to support the Financial Operating Plan process. We will share best practices on how this data can be utilized by Project Leadership at both the sponsor and service provider to manage resource assignment for operational delivery of the associated studies, monitor performance and provide reliable forecast data utilizing activity based methodologies.

5:45 Reception hosted by Exostar
Download Brochure
Thursday, January 26
7:15 am Registration
7:30 Co-Breakfast Presentation: The Inspiring Hope Ideathon: Solutioning the Clinical Trial Awareness Gap
 Christine Phillips, Senior Director, Site & Patient Access, INC Research
Christine Phillips, Senior Director, Site & Patient Access, INC Research
 Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
To advance society’s ability to respond to future healthcare challenges and advance medical innovation we must increase awareness of clinical research and study participation. Clinical research is vital to the development of new drugs and treatments but is dependent on patient participation. The “Inspiring Hope Ideathon” was the first initiative of its kind designed to generate new and unique ideas. The participation and results were groundbreaking and will be shared here!
8:35 Chairperson’s Remarks
Rick Morrison, CEO, Comprehend
8:40 Like Mars & Venus...Only Worse: Exploration of Common Peeves in the Sponsor/Service Provider Relationships and Potential Ways to Mitigate
 Chris Chan, Senior Director, R&D Finance, FibroGen, Inc.
Chris Chan, Senior Director, R&D Finance, FibroGen, Inc.
The sponsor/vendor relationship has been an ongoing issue and challenge throughout the ongoing history of outsourced drug development activities. This presentation will identify some of these issues and discuss ways to alleviate.
9:05 So I Contracted with My Vendor…Now What?!
 Tenley Koepnick, Senior Director, Clinical Operations, Edwards Lifesciences
Tenley Koepnick, Senior Director, Clinical Operations, Edwards Lifesciences
The development of an ongoing, iterative, and collaborative vendor oversight dialog is a natural and needed progression following the outsourcing and contract execution process. This session will explore the often under-valued, yet GCP-required need for a Sponsor to maintain proper vendor oversight. Even more so, we will explore practical techniques that a Sponsor or Vendor could implement to drive the quality of vendor deliverables throughout the contract duration. Focus will be on building positive Sponsor-Vendor relationships with examples of what has worked (…and what has not worked).
9:30 Evaluating Sponsor-Service Provider Relationships and Performance
 Elspeth Carnan, Global Head, R&D Operational Excellence, Sunovion Pharmaceuticals
Elspeth Carnan, Global Head, R&D Operational Excellence, Sunovion Pharmaceuticals
9:55 A Data-Driven Approach to More Effective Studies Strategies
 G. Paul Evans, Ph.D., Corporate Vice President, Global Site Solutions, PAREXEL International
G. Paul Evans, Ph.D., Corporate Vice President, Global Site Solutions, PAREXEL International
A discussion on a data-driven method for applying actionable intelligence and predictive analytics to produce more effective study strategies. Transforming data into knowledge can be applied in various arenas ranging from protocol design, country and site selection, site access, patient enrollment, quality, and satisfaction. The data can ultimately improve feasibility, drive better enrollment solutions, and mitigate risk.
10:20 Coffee Break
10:35 Chairperson’s Remarks
John Boland, Vice President, Product Development, Atlantic Research Group, Inc.
10:40 CO-PRESENTATION: A Model Methodology for Building a Win-Win Partnership between a Sponsor and CRO
 Mark Mann, Head, Clinical Outsourcing and Contracts, Upsher-Smith Laboratories
Mark Mann, Head, Clinical Outsourcing and Contracts, Upsher-Smith Laboratories
 Julie Ross, President, Advanced Clinical
Julie Ross, President, Advanced Clinical
The model methodology for building a win-win partnership between a Sponsor and CRO starts with trust and vulnerability. You need to break down barriers and build behaviors and actions which will determine desired outcomes. Each organization not only needs to track performance and deliverables, they need to track the “right stuff”, because true success is measured beyond contract deliverables.
11:20 Sponsored Presentation (Opportunity Available)
11:35 A Method for Establishing a Set of Sponsor & Vendor Oversight Metrics
 Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
The model followed by today’s sponsors is to outsource most, if not all, functional area study tasks to vendors. Sponsor resources no longer do; they manage. This session provides the steps for establishing effective metrics for managing oversight; metrics that matter and that report results at the appropriate oversight level. We will outline the use of a Clinical Trial Process Map, Critical Success Factors, Key Performance Questions and the proper application of metric visualizations to communicate result trends when developing and reporting oversight metrics.
12:00 pm PANEL DISCUSSION: Determining a Good Sponsor/Vendor Partnership
 Réne Stephens, Executive Director, Global Head, Global Contracts & Outsourcing Management (GCOM), Astellas
Réne Stephens, Executive Director, Global Head, Global Contracts & Outsourcing Management (GCOM), Astellas
 Tenley Koepnick, Senior Director, Clinical Operations, Edwards Lifesciences
Tenley Koepnick, Senior Director, Clinical Operations, Edwards Lifesciences
 Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
 Chris Chan, Senior Director, R&D Finance, FibroGen, Inc.
Chris Chan, Senior Director, R&D Finance, FibroGen, Inc.
 Andrew Townshend, Senior Vice President, Alliance Development, INC Research
Andrew Townshend, Senior Vice President, Alliance Development, INC Research
 Charlotte French, Senior Director, Global Head, Contracting & Outsourcing, EMD Serono
Charlotte French, Senior Director, Global Head, Contracting & Outsourcing, EMD Serono
Christopher Rull, Vice President, Head of Business Development & Account Management, UBC
Luke Van Hengel, Corporate Vice President, Business Operations, PAREXEL International
The panel will discuss each function’s role and perspective on how they measure and evaluate a good sponsor-vendor relationship and how the group factors each function’s perspective to make decisions that affect the sponsor-vendor relationship.
12:50 Closing Remarks
12:55 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
SCOPE 2016 Wrap-Up
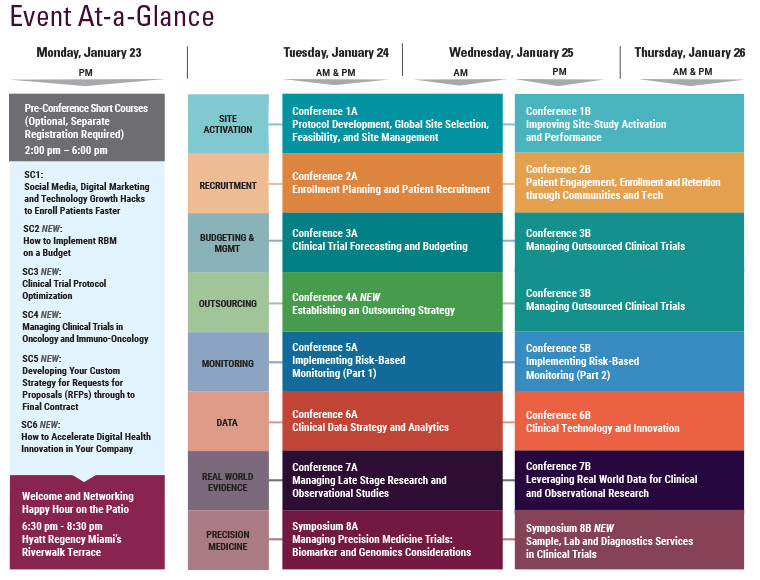
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Lee Yuan
Conference Producer
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com