Cambridge Healthtech Institute’s 10th Annual
Enrollment Planning and Patient Recruitment:
Successful Recruitment Planning, Forecasting, and Central Campaign Management
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
Patient recruitment and up-front enrollment planning are critical to drug development programs. Patient recruitment, if not adequately planned for, can extend your development timeline by a number of years. Retention of patients throughout the life of a clinical trial is essential in order to have complete data sets for your analysis and subsequent filings. In order to optimize both, you have to have a plan and it should take into account all stakeholders from senior management at the sponsor company and the CRO partners, to the sites and investigators and study volunteers. Cambridge Healthtech Institute’s Tenth Annual "Enrollment Planning and Patient Recruitment" conference will cover the topics one should consider when drafting and strategically implementing a patient recruitment plan for a clinical development program.
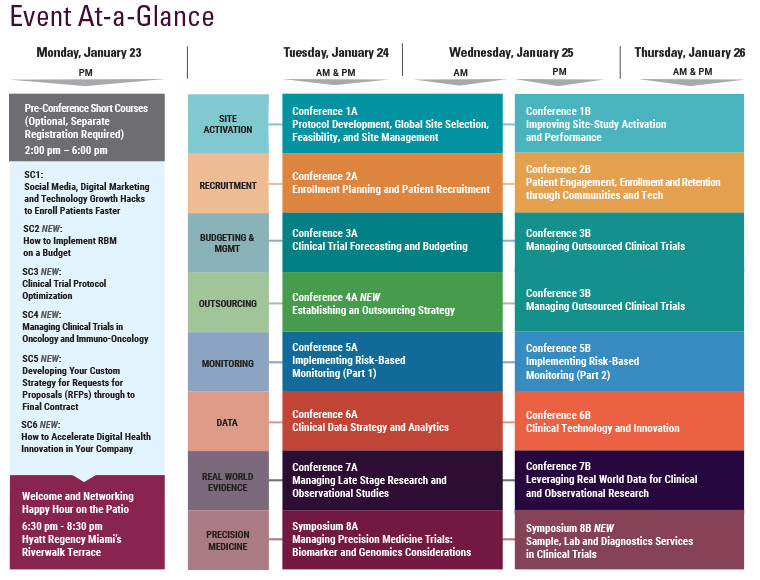
Final Agenda
Monday, January 23
1:00 pm Short Course Registration
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Kelly McKee, Advisor, Clinical Innovation, Eli Lilly and Co.
10:50 Pfizer’s Approach to Improving Operational Predictability
 Mohanish Anand, Ph.D., Senior Director and Head, Feasibility Center of Excellence, Development Operations, Pfizer
Mohanish Anand, Ph.D., Senior Director and Head, Feasibility Center of Excellence, Development Operations, Pfizer
Pfizer Feasibility Center of Excellence has been working to leverage data & advanced analytics & data visualization in order to help enable the predictive delivery of the portfolio. This represents an innovative approach to enrollment forecasting challenges that all sponsor companies face. The speaker will share an overview of some of these efforts and early results. Questions from audience members will be encouraged.
11:15 Moving Recruitment Planning Upstream to Reduce Barriers to Participation: Recommendations from the CTTI Recruitment Planning Project
 Beth Mahon, Associate Director, Global Clinical Operations - US, Janssen Research and Development
Beth Mahon, Associate Director, Global Clinical Operations - US, Janssen Research and Development
As many as 85% of studies are not completed on time because of recruitment and as many as 30% of sites do not even recruit one patient. This session will include a presentation of key findings and themes from our evidence gathering and consensus building processes that led to recommendations and a systematic framework for moving recruitment planning upstream in the protocol design and development process. These findings include a systematic literature review, multi-stakeholder survey, landscape scan of available recruitment planning tools and processes, and consensus generated at multi-stakeholder expert meeting.
11:40 Innovative Patient Transportation Options to Increasing Retention: Facilitating the New Paradigm of Patient-Centered Trials
 Nariman Nasser, Vice President, Site Optimization, Continuum Clinical
Nariman Nasser, Vice President, Site Optimization, Continuum Clinical
Sites and patients often struggle with determining the most efficient and cost effective mode of transportation to attend study visits. Utilizing ridesharing solutions represents a new solution to meet the site and patient needs around safety, security, reliability, and compliance. This disruptive innovation is making its way into our industry, reducing the burden of site reimbursement processes for transportation costs and decreasing study visit no-shows.
 12:05 pm Patient Recruitment: Sponsor Insights
12:05 pm Patient Recruitment: Sponsor Insights
 Melynda Geurts, Vice President, Operations, Imperial
Melynda Geurts, Vice President, Operations, Imperial
Subject enrollment is a widespread issue in clinical trials, adding significant expense and time delays to the overall scope of studies. We surveyed sponsor operations management personnel to gain insight and assess sponsor attitudes and behaviors in conducting clinical trials. We will identify current decision-making and accountability roles, enrollment metrics, stressors, and tactics with regards to patient recruitment at sponsor companies.
 12:40 Luncheon Co-Presentation: Leveraging Digital and Social Media: How Patient Insights and Discoverable Data Can Lead to More Effective and Efficient Patient Recruitment and Retention Campaigns
12:40 Luncheon Co-Presentation: Leveraging Digital and Social Media: How Patient Insights and Discoverable Data Can Lead to More Effective and Efficient Patient Recruitment and Retention Campaigns
 Robert Loll, Senior Vice President, Business Development & Strategic Planning, Praxis
Robert Loll, Senior Vice President, Business Development & Strategic Planning, Praxis
 Tricia Barrett, Senior Vice President, Managing Director, Praxis
Tricia Barrett, Senior Vice President, Managing Director, Praxis
As the world becomes more and more digital, it is important to tap into the endless amounts of data living and being shared on the Internet. As we have lunch, we’ll explore how our industry can utilize this data to determine what is useful and relevant, and then glean insights and understanding before crafting patient-centric enrollment campaigns.
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Robert Loll, Vice President, Business Development & Strategic Planning, Praxis
2:05 Understanding FDA Requirements and Implementing the New Reality of Diversity in Clinical Trials
 Karen Brooks, Ph.D., Senior Director, Clinical Operations, Adare Pharmaceuticals
Karen Brooks, Ph.D., Senior Director, Clinical Operations, Adare Pharmaceuticals
Understand regulatory changes from FDA and updated requirements for ethnicity/race inclusion in trial populations. How do you formalize into a clinical development plan at a company level to make it part of corporate culture by educating and training teams so that they can embrace the ethnicity value? How do you then implement at project team level and operationalize the activities to support diversity in clinical research.
2:30 CASE STUDY AND INTERACTIVE PANEL: Improving Patient Diversity in Clinical Trials with Real-Time Enrollment Monitoring
One of the largest problems we are facing when it comes to clinical trial participation is the lack of diversity in our enrollment demographics. The vast majority of trials are dominated by white participants, and it is a trend that has not gone unnoticed by the FDA and other regulatory authorities, who rightly want to see pharma make major strides in improving patient diversity. This session will begin with the Merck case study presentation on their latest Hep-C program and segue into a targeted panel discussion about improving diversity and recruitment.
2:30 CASE STUDY: Operationalizing Diversity Initiatives in Clinical Research, a Hep-C Story
 Marisa Rackley, Director, Clinical Research, Global Trial Optimization, Merck
Marisa Rackley, Director, Clinical Research, Global Trial Optimization, Merck
The industry understands that diversity in clinical trial participation is important. Translating those values into operational plans for particular trials has been a challenge. Building a framework for teams is critical in order to realize installation of these corporate initiatives. As Hep-C does impact minority populations, it was critical that the trial reflect these demographics and improve upon previous statistics (for example, 1-3% African American participation). In order to achieve their goals, Merck launched a comprehensive strategy leveraging best practices in strategic site selection, patient-facing materials and a real-time enrollment monitoring tool. The results were impressive with a 15% African American participation rate.
2:55 INTERACTIVE PANEL: Improving Patient Diversity in Clinical Trials with Real-Time Enrollment Monitoring
Moderator:
 Robert Loll, Vice President, Business Development & Strategic Planning, Praxis
Robert Loll, Vice President, Business Development & Strategic Planning, Praxis
 Fabian Sandoval, M.D., CEO & Medical Director, Emerson Clinical Research Institute (ECRI)
Fabian Sandoval, M.D., CEO & Medical Director, Emerson Clinical Research Institute (ECRI)
 Marisa Rackley, Director, Clinical Research, Global Trial Optimization, Merck
Marisa Rackley, Director, Clinical Research, Global Trial Optimization, Merck

Karen Brooks, Ph.D., Senior Director, Clinical Operations, Adare Pharmaceuticals
This panel will discuss the lack of diversity in our enrollment demographics and address these key points in an interactive format:
- Understand the current state of diversity and why it’s important to the FDA
- Review Merck’s successful Hep-C program and how it worked to achieve these goals
- Learn how to operationalize the activities to support diversity in clinical trials
- Learn from sponsor, site and vendor perspectives how new techniques can make a big difference – through site selection, patient recruitment, site management and technology tools
3:20 Accelerate Clinical Trial Recruitment and Engage HealthCare Providers as Referral Sources with Specialized Clinical Field Resources
 Stewart Rosen, M.D., Vice President of Medical Affairs, Quintiles Health Management Solutions, QuintilesIMS
Stewart Rosen, M.D., Vice President of Medical Affairs, Quintiles Health Management Solutions, QuintilesIMS
Speed and efficiency have never been more critical in clinical studies. Researchers need to consider new avenues to compress timelines. Even with careful planning, potential barriers can derail study schedules at any stage and impact results. Competition for sites may be intense, slowing the selection process. Activated sites may fail to focus aggressively on patient enrollment or lack the network to find a particular patient population. Specialized Clinical Field Resources such as Clinical Trial Educators can accelerate enrollment, improve site performance and be a primary source to educated referral networks on behalf of sites.
3:45 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, January 25
7:15 am Registration
7:30 Breakfast Presentation: eConsent: Put "Informed" Back in Informed Patient Consent
 Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Electronic informed consent makes the consenting process more efficient and effective for staff, sponsors, monitors, and most importantly patients by presenting the information in formats and language in which patients are comfortable. Join us for breakfast to learn the best practices, latest technological advances, and proven benefits of deploying an eConsent solution - including the impact it has on improving patient satisfaction and retention and help us put "informed" back in patient consent process.
8:25 Chairperson’s Remarks
Mark Summers, CEO & President, ThreeWire, Inc.
8:30 Overcoming Recruitment Challenges in Rare Disease
 Elizabeth Carfioli, Associate Director, Patient Recruitment, Clinical Operations, Alnylam Pharmaceuticals
Elizabeth Carfioli, Associate Director, Patient Recruitment, Clinical Operations, Alnylam Pharmaceuticals
Patient recruitment is challenging, even more so in Rare Disease. This presentation will share recent approaches undertaken at Alnylam Pharmaceuticals, in collaboration with our development partnership and with CROs, to achieve global recruitment goals. We will discuss country and site tailored strategies to address disease awareness to meet not only recruitment goals but longer-term strategies to identify potential patients for future trials through commercialization and share how we collaborate and engage with our sites to bring patients, their families and trials together.
8:55 Increasing Recruitment and Retention through Comprehensive Patient Engagement & Patient Advisory Boards (PABs)
 Tanja Keiper, Ph.D., Director, Global Clinical Operations - External Innovation, EMD Serono, Inc.
Tanja Keiper, Ph.D., Director, Global Clinical Operations - External Innovation, EMD Serono, Inc.
Co-developed by Paulo Moreira, Vice President, Global Clinical Operations - External Innovation, EMD Serono, Inc.
Struggles with recruitment and retention and efforts focused to address these issues have often overlooked a key objective: bi-directional engagement with patients. This presentation will share solutions for addressing these gaps. Also, the talk will discuss the key role of Patient Advisory Boards (PABs) in clinical development.
 9:20 Co-Presentation: Starting Recruitment Before the Site Initiation Visit: 10 Steps
9:20 Co-Presentation: Starting Recruitment Before the Site Initiation Visit: 10 Steps
Heidi Ross, RN, BSN, Project Director, Patient & Physician Services, UBC
LaShell Robinson, MS, Manager, Patient & Physician Services, UBC
This presentation will cover: 1) Reducing time from site activation to first patient enrolled 2) Pre-enrollment activities – building the repository of eligible patients 3) Priming sites for early patient identification and meeting enrollment goals 4) Identifying and mitigating site-specific pitfalls to patient enrollment
9:45 SCOPE’s 2017 Participant Engagement Award
brought to you by CHI’s SCOPE and Patient Enrollment Advisors
The 2017 Participant Engagement Award is designed to inspire innovation and change in how we communicate to participants in the fields of Recruitment and Retention for clinical trials. Cambridge Healthtech Institute (CHI)’s SCOPE and Patient Enrollment Advisors welcome submissions from every aspect of the industry including Sites, CRO’s, Agencies and Sponsors alike to submit their best work in the Patient Recruitment and Retention communications field.
Panel of Judges:
David Sall, President & CEO, Patient Enrollment Advisors
Kelly McKee, Advisor, Clinical Innovation, Eli Lilly and Co.
Jean-Christian Philippi, Founder and Chief Strategy Officer, One Creative
Beth Mahon, Associate Director, Global Clinical Operations - US, Janssen Research and Development
Mark Sloan, M.D., Hematology & Medical Oncology, Boston Medical Center
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Gretchen Goller, MSW, Senior Director, PARS, PRA Health Sciences
11:15 FEATURED PRESENTATION: Bringining the Patient Voice and Community into the Drug Development Process
 Francis Kalush, Ph.D., Health Programs Coordinator, Professional Affairs and Stakeholder Engagement (PASE), CDER, FDA
Francis Kalush, Ph.D., Health Programs Coordinator, Professional Affairs and Stakeholder Engagement (PASE), CDER, FDA
FDA’s Professional Affairs and Stakeholder Engagement at CDER is has been working with all stakeholders: patients/patient advocates, health professionals and industry to bring the patient voice and perspective into the drug development and approval process. The goal would be to continue the support of novel therapies that directly address the need of patients living with diseases. Learn to effectively engage with FDA. Understand how to bring the voice of the patient into the drug approval process.
11:40 INTERACTIVE PANEL: Recruitment in a Resource Constrained Environment: Do Past Tactics Still Give the Same Outcome in Present Day Scenarios?
Moderator:
 Jeffrey Kasher, Ph.D., President, Patients Can't Wait
Jeffrey Kasher, Ph.D., President, Patients Can't Wait
 Nariman Nasser, Vice President, Site Optimization, Continuum Clinical
Nariman Nasser, Vice President, Site Optimization, Continuum Clinical
 Madeline Geday, Associate Director, Clinical Research, Patient and Stakeholder Engagement Lead, Global Trial Optimization Group, Merck & Co
Madeline Geday, Associate Director, Clinical Research, Patient and Stakeholder Engagement Lead, Global Trial Optimization Group, Merck & Co
 Mohanish Anand, Ph.D., Senior Director and Head, Feasibility Center of Excellence, Development Operations, Pfizer
Mohanish Anand, Ph.D., Senior Director and Head, Feasibility Center of Excellence, Development Operations, Pfizer
An introspective panel looking at the current landscape we face when trying to enroll patients in a study. Panelists will provide 2 scenarios outlining the tactics used to support the trial, while involving the audience to further the discussion. Topics such as Patient Engagement, increased need for justification in site selections by regulatory authorities, as well as decreased recruitment dollars will be discussed.
- Reinforcement of tactics potentially already known, but potentially not selected due to potential archaic nature
- Introduction to tactics not known or considered
- Industry perspective into how similar all of our trial concerns are
- Help vendors also understand that more must be done with less and bidding may be significantly lower than typical
12:05 Bridging Luncheon Presentation: A Breakthrough in Technology Enabled Clinical Trials Recruitment
 Steven Coca, M.D., Associate Professor of Medicine, Internal Medicine, Icahn School of Medicine, Mount Sinai
Steven Coca, M.D., Associate Professor of Medicine, Internal Medicine, Icahn School of Medicine, Mount Sinai
Dr. Coca is presenting on the effectiveness of CLiX ENRICH in accelerating patient recruitment illustrated by two case studies: a retroactive analysis of a trial in which CLiX ENRICH yielded twice the candidates in half the time; and a live trial whereby CLiX identified a large cohort of quality patients.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com