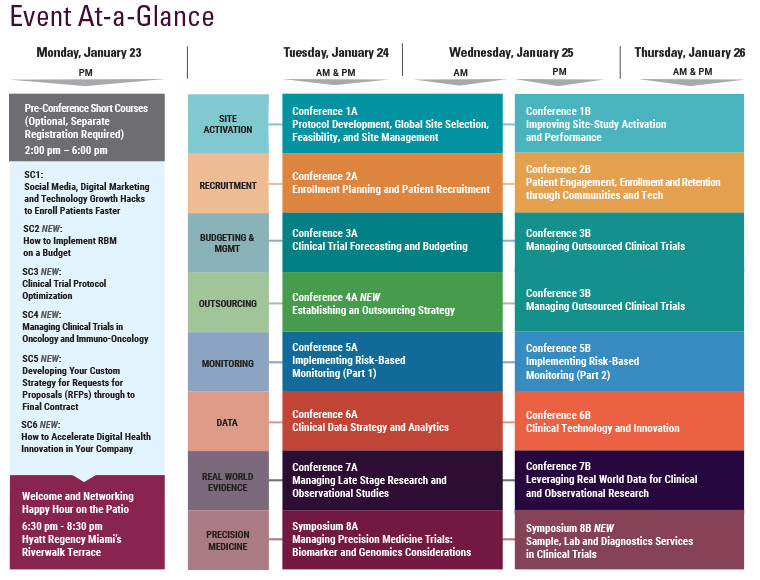
Cambridge Healthtech Institute’s 3rd Annual
Implementing Risk-Based Monitoring – Part 2:
Ensuring Effective and Efficient Monitoring and Data Quality
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
Risk-based monitoring (RBM) approaches promise to improve clinical trial efficiency while ensuring data quality. As industry adoption of RBM increases, it is clear that successful risk-based monitoring implementation requires developing new roles, analytics and processes among the stakeholders in RBM. Cambridge Healthtech Institute’s Third Annual "Implementing Risk-Based Monitoring – Part 2: Ensuring Effective and Efficient Monitoring and Data Quality" conference offers case studies and practical solutions from across pharma and TransCelerate member organizations on effectively working with various stakeholders in RBM as well as leveraging technology to benefit RBM.
Final Agenda
Wednesday, January 25
12:05 pm Bridging Luncheon Presentation: Risk-Based Monitoring – Making it Work
 Keith Howells, Senior Vice President, Development, OmniComm Systems, Inc.
Keith Howells, Senior Vice President, Development, OmniComm Systems, Inc.
A major benefit of risk-based monitoring is to perform less than 100% Source Document Verification, freeing monitoring staff for more valuable work and reducing the burden on the sites. We will share an example of the new workflow during a monitoring visit, analyze trials where RBM have been implemented and identify specific approaches that have been employed.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairpersons’ Remarks
Brian Nugent, Director, Clinical Operations & Process, Gilead Sciences &
Angie Maurer, RN, BSN, MBA, Clinical Operations Consultant, PALM/Clinical Operations, Gilead Sciences
4:05 RBM Change Management Successes: Stories and Lessons Learned
Andy Lawton, Director & Consultant, Risk Based Approach Ltd.
Every organization has not only its own systems and processes surrounding the introduction of RBM, but also its management strategy and tactics directing the transformation. In this summary of research conducted with organizations implementing RBM, we identify both common themes and unique change management challenges that organizations encounter, the techniques and tools employed, and the lessons learned along the way. We will share success (and failure) stories that have been shared with us so that others may capitalize upon (or avoid) these during their own journeys implementing RBM.
 4:30 Co-Presentation: An Innovative Approach to RBM Success: A Strategic Solution for Sponsors, CROs and Sites
4:30 Co-Presentation: An Innovative Approach to RBM Success: A Strategic Solution for Sponsors, CROs and Sites
 Mary Mills, RN, CCRA, Consultant Expert, Risk Based Monitoring Strategy, SDS Clinical LLC
Mary Mills, RN, CCRA, Consultant Expert, Risk Based Monitoring Strategy, SDS Clinical LLC
 Jane Shen, Pharm.D., Senior Director, Innovation, PMG Research
Jane Shen, Pharm.D., Senior Director, Innovation, PMG Research
This session presents an innovative approach to the implementation of RBM by including risk management planning for the trial at the site level. This original strategy of partnering with sites at the time of the study feasibility, of defining the quality metrics and the reporting frequency needed for sites to implement their quality systems, successfully drove performance during the trial. It will be increasingly important with the deployment of ICH GCP E6(R2).
4:55 PANEL DISCUSSION: Bringing Together Key Stakeholders in RBM
 Brian Nugent, Director, Clinical Operations & Process, Gilead Sciences
Brian Nugent, Director, Clinical Operations & Process, Gilead Sciences
 Angie Maurer, RN, BSN, MBA, Clinical Operations Consultant, PALM/Clinical Operations, Gilead Sciences
Angie Maurer, RN, BSN, MBA, Clinical Operations Consultant, PALM/Clinical Operations, Gilead Sciences
 Justin Stark, Director/Head, Risk Based Monitoring, UCB Biosciences, Inc.
Justin Stark, Director/Head, Risk Based Monitoring, UCB Biosciences, Inc.
 Ken Wu, Clinical Operations Consultant, Kenneth Wu and Associates, LLC
Ken Wu, Clinical Operations Consultant, Kenneth Wu and Associates, LLC
 Kristin Mauri, MBA, PMP, Senior Director, Global Consulting, eHealth Solutions, Bioclinica
Kristin Mauri, MBA, PMP, Senior Director, Global Consulting, eHealth Solutions, Bioclinica
 Michael Walega, Executive Director, Monitoring and Data Flow Optimization, Clinical Development Services, Covance, Inc.
Michael Walega, Executive Director, Monitoring and Data Flow Optimization, Clinical Development Services, Covance, Inc.
Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, PPD
The panel will discuss, from a pharma and CRO perspective, creating and organizing a successful clinical ops team around implementing risk-based monitoring and the delicate balance in deciding what is essential vs. nice to have for an organization of your size, whether small, mid-size or large.
5:45 Close of Day
Download Brochure
Thursday, January 26
7:15 am Registration
7:30 Co-Breakfast Presentation: The Inspiring Hope Ideathon: Solutioning the Clinical Trial Awareness Gap
 Christine Phillips, Senior Director, Site & Patient Access, INC Research
Christine Phillips, Senior Director, Site & Patient Access, INC Research
 Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
To advance society’s ability to respond to future healthcare challenges and advance medical innovation we must increase awareness of clinical research and study participation. Clinical research is vital to the development of new drugs and treatments but is dependent on patient participation. The “Inspiring Hope Ideathon” was the first initiative of its kind designed to generate new and unique ideas. The participation and results were groundbreaking and will be shared here!
8:35 Chairperson’s Remarks
Debra Jendrasek, Vice President, Strategic Development Partner, SA & SD, Strategic Development, Chiltern
8:40 Risk-Based Monitoring (RBM) Case Study
 Lynne Cesario, Risk Based Monitoring Program Lead, Clinical Sciences and Operations, Global Product Development, Pfizer
Lynne Cesario, Risk Based Monitoring Program Lead, Clinical Sciences and Operations, Global Product Development, Pfizer
Recent draft updates to the ICH E6 GCP Guidance will require sponsor companies to incorporate sound quality risk management (QRM) approaches into every clinical study. How do companies convene key stakeholders, optimize resources and ultimately implement this? This presentation will share examples and key learnings from our ongoing work in Risk-Based Monitoring (RBM).
9:05 Designing and Implementing an Analytical Risk-Based Model at Janssen R&D
 Stephanie Clark, Associate Director, Risk Management-Central Monitoring, Janssen Research & Development, LLC
Stephanie Clark, Associate Director, Risk Management-Central Monitoring, Janssen Research & Development, LLC
RBM implementation is probably one of the most complex endeavors the industry is trying to tackle since the advent of EDC in the 90’s. There are several cross-roads that companies encounter as they embark on the RBM journey, and for each organization there are unique challenges. However, in many cases the key components of a successful RBM program are universal. Join as Stephanie shares her insights on the Janssen experience in designing and implementing an Analytical Risk-Based model of study management and monitoring across the company portfolio.
9:30 Co-Presentation: Lessons Learned from RBM Deployments
 Jacqueline Gough, Advisor, Clinical Risk Management, Eli Lilly and Company
Jacqueline Gough, Advisor, Clinical Risk Management, Eli Lilly and Company
 Michael Walega, Executive Director, Monitoring and Data Flow Optimization, Clinical Development Services, Covance, Inc.
Michael Walega, Executive Director, Monitoring and Data Flow Optimization, Clinical Development Services, Covance, Inc.
Risk-Based Monitoring (RBM) is changing the way companies are conducting clinical trials. Implementing RBM requires companies to re-evaluate processes and tools for monitoring as well as how they perform their early trial planning. The key decision points in the building and deployment of an RBM capability will be discussed as well as the most common pitfalls.
9:55 Making RBM Work for Your Organization
 Karen McCarthy Schau, Principal Consultant, Clinical Optimization Practice, Paragon Solutions
Karen McCarthy Schau, Principal Consultant, Clinical Optimization Practice, Paragon Solutions
It can be overwhelming to think about everything that needs to occur prior to deploying an RBM solution. We will provide a framework that will guide your deployment planning. Additionally, we will discuss trends/best practices for each component of the solution (technology, data, organizational design (Sponsor and CROs), processes, and governance). At the end of this session, you should feel more comfortable as you begin your deployment journey.
10:20 Coffee Break
10:35 Chairperson’s Remarks
Francois Torche, CEO, CluePoints
 10:40 Implementing Risk-Based Monitoring Solutions from a System and Architecture Perspective
10:40 Implementing Risk-Based Monitoring Solutions from a System and Architecture Perspective
 Michael Bem, IT Manager, Medicines Development, Eli Lilly and Company
Michael Bem, IT Manager, Medicines Development, Eli Lilly and Company
.jpg) Alan Switzer, MBA, Director, IT Business Initiatives, Covance
Alan Switzer, MBA, Director, IT Business Initiatives, Covance
Through the use of quality and risk approaches into the scientific design and operational conduct of clinical trials, risk can be mitigated and issues can be detected early or prevented entirely. Lilly initially implemented a risk-based monitoring solution through integrated data flows with internally built visualizations. In addition, we have partnered with a third party to implement our long term solutions via a third party provider as a software as a service. This talk will discuss the technical approaches and architectural challenges we have used to build the solutions with our internal solution and with our third party.
11:35 pm Searching for a Technology Solution to Support Risk-Based Monitoring (RBM)
 Ed Kellar, Director, Global Data Management Operational Support, Astellas, Inc.
Ed Kellar, Director, Global Data Management Operational Support, Astellas, Inc.
TransCelerate published “Position Paper: Risk-Based Monitoring Methodology” in May 2013. They proposed transformational process improvements in industry monitoring practices utilizing a methodology based on quality risk management, designed to both increase efficiency and enhance data integrity while maintaining adherence to good clinical practice. In 2014, the TransCelerate RBM Technology Sub-Team followed this paper with a White Paper, “Technology Considerations to Enable the Risk-Based Monitoring Methodology”, which provided a description of the technical capabilities needed to support RBM. Now, the RBM Technology Sub-Team has published a second White Paper, “TransCelerate Risk-Based Monitoring Technology Considerations Part 2” which builds on previous work and provides a thorough set of specific system considerations for a RBM platform that includes people, process and technology perspectives. This presentation will describe the steps taken by the TransCelerate Technology Sub-Team to elicit, analyze and publish this comprehensive set of user and system considerations and the business use cases that drive them, with input from the vendor community and member companies. In addition, we will review vendor provided recommendations and case studies from TransCelerate member companies and discuss the current state of RBM technology solutions.
12:00 pm CoLAB: Redefining Collaborative Engagement with Patients in Clinical Trials
 Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
The purpose of CoLAB is to improve Lilly clinical trials by considering the Site, Patient, and Patient-partner perspective. Site and Patient Simulation is one of the ways that CoLAB brings together Lilly study teams, clinical site Study Coordinators, Patients, and Patient-partners to understand real-world feedback on operational issues within our clinical protocols. By engaging your Patients upfront, you can ensure that good science aligns with good patient care. By engaging Patients early in protocol development, you can potentially improve the clinical research patient experience.
12:25 CO-PRESENTATION: Engaging with Sites and Patients to Enable Digital Innovation for Clinical Trials
 Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
 Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
In the new healthcare ecosystem and digital age, patients expect care and solutions that are coordinated, convenient, customized, and accessible. Biopharmaceutical companies are doing a lot to address these emerging expectations for patient engagement services and we are all learning a lot on the way. It is important to truly engage with sites, investigators and research volunteers using both traditional and hi-tech means and to learn from those early and ongoing interactions. With Aspire, a unique BMS effort that will be shared in this presentation, we put the focus on the Sites and Patients and the results are guiding other trial planning and management efforts.
12:50 INTERACTIVE PANEL: Digital Clinical Trial Lessons Learned: Panel Discussion from Pharma Innovators Who Have Run Virtual Trials
Moderator:
 Matt Hendricks, Partner, Pharmica Consulting
Matt Hendricks, Partner, Pharmica Consulting
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer-Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer-Ingelheim
 Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
 Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
 Debbie Profit, Ph.D., Leader, IT, Otsuka
Debbie Profit, Ph.D., Leader, IT, Otsuka
In the past year, several large Pharma companies have begun experimenting with a new breed of reimagined clinical trials which leverage wearables and fewer sites. Now the results from the first round of these experiments are in, and the pioneers who ran the studies are ready to share their findings. Join us as we discuss what aspects of these studies are ready for prime time, where there is still work to be done, and most importantly, how patients have reacted to this shift. The conversation will focus on platforms & technology from industry veterans, startups, and established newcomers such as Apple and their ResearchKit platform.
1:15 Closing Remarks
1:20 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Lee Yuan
Conference Director
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com