Cambridge Healthtech Institute’s 3rd Annual
Leveraging Real World Data for Clinical and Observational Research:
Integrating Evidence Generation with RWD and Pre-Existing Data
February 14-15, 2018 | Hyatt Regency Orlando | Orlando, FL
The abundance of data generated during routine health care is growing in significance and should be re-used for clinical and observational research. Patient electronic records, registries, data from pharmacy and social media, and wearable sensors have
been increasingly used as eSources. This process requires strategizing, utilizing novel data technologies, as well as close collaboration between pharmaceutical companies and organizations that possess the data. CHI’s 3rd Annual Leveraging Real World Data for Clinical and Observational Research will
discuss challenges and solutions with secondary use of existing healthcare data for assessing the effectiveness and safety of medical products.
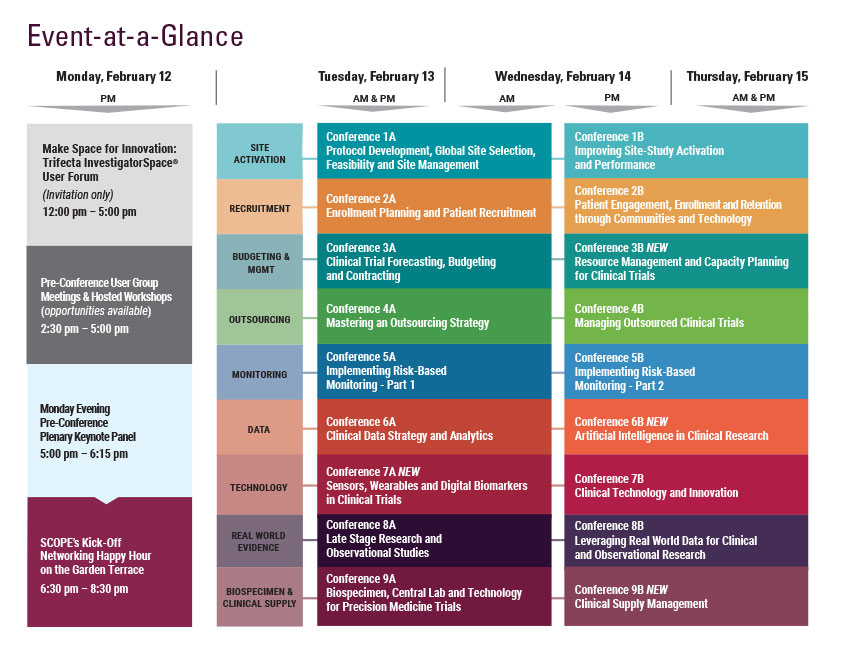
Wednesday, February 14
11:30 am Registration Open
 12:10
pm Bridging Luncheon Presentation: Achieving Evidentiary Equilibrium
12:10
pm Bridging Luncheon Presentation: Achieving Evidentiary Equilibrium
 David Thompson, Ph.D., Senior Vice President, Real-World & Late Phase, Syneos Health
David Thompson, Ph.D., Senior Vice President, Real-World & Late Phase, Syneos Health
Achieving Evidentiary Equilibrium - Generating the Right Evidence for the Right Stakeholders at the Right Time Throughout the Clinical/Commercial Continuum
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Valentine’s Day Celebration in the Exhibit Hall, Last Chance for Exhibit Viewing
4:00 Chairperson’s Remarks
Sean Mooney, Ph.D., Chief Research Information Officer, UW Medicine
4:05 Systematic Approach to Use RWD to Inform Trial Design: Going beyond Simple Feasibility
 Hui Cao, M.D., Ph.D., Executive Director, Real-World Evidence, COE for RWE, Global Medical Affairs, Novartis Pharmaceuticals Corporation
Hui Cao, M.D., Ph.D., Executive Director, Real-World Evidence, COE for RWE, Global Medical Affairs, Novartis Pharmaceuticals Corporation
We developed a structured framework to guide the use of RWD to inform clinical trial design. This framework consists of three dimensions: recruitability, efficacy endpoint impact and risk impact. Each major criterion in the trial inclusion/exclusion criteria
can be assessed on how it would impact the size of patient pool, the efficacy endpoints and risks. This framework was validated via its application on pre-authorization pivotal trials.
4:30 RWE Analytics to Link Patient Journey with Trial Feasibility and Patient Recruitment
 Jane Fang, M.D., Head, Research & Development Information for Clinical Biologics, MedImmune Biologics Science Unit of AstraZeneca
Jane Fang, M.D., Head, Research & Development Information for Clinical Biologics, MedImmune Biologics Science Unit of AstraZeneca
The precision medicine behind trial design is driving rapid change in the way of traditional feasibility assessment and patient recruitment. Emerging market of healthcare information and records providers is bringing game-changing approaches to enable
hypothesis generation of protocol design, deep dive analysis of patient journey and precision patient recruitment. The innovative approach of linking RWE analysis with clinical trial research and patient recruitment is critical for today and future
precision trial conduct.
4:55 Leveraging Real World Data to Streamline Clinical Trials
Manfred Stapff, M.D., Ph.D., CMO, TriNetX, Inc.
Industry is encumbered with lengthy drug development timelines and high costs. These challenges are correlated with the difficulty to recruit appropriate patient populations for clinical trials. Join this session to learn how real world data is
transforming the way biopharma, CROs and study sites navigate obstacles in drug development across protocol design, site selection and patient recruitment.
5:25 Current State of Real World Evidence to Support the Medicine Lifecycle
Nicolle Gatto, Group Lead, Senior Director, Epidemiology, Worldwide Safety and Regulatory, Pfizer
Observational data from electronic health records, insurance claims and disease registries are often used for premarketing studies. This presentation will describe the uses of real-world, observational data across the drug development lifecycle
with particular focus on more innovative uses such as serving as a source for embedded clinical trials, for active surveillance of rare events across multiple linked data sources where relevant, case studies will be briefly described.
5:50 Close of Day
5:50 - 7:00 pm Track Reception (Sponsorship Opportunity Available)
Thursday, February 15
7:15 am Registration Open
 7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
7:45 Breakfast Presentation: Clinical Trial Operations Insights Evolved via Accenture Life Sciences Cloud
 Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chandi Kodthiwada, Product Manager, Accenture Life Sciences Cloud (ALSC), Accenture
Chances are you have spent a meaningful amount of time on one of the below questions: How do you assess your Clinical Trial operational efficiency? How do I stay on top of Site behavior? How do you maintain Vendor-Oversight? In the presentation,
we will go through Accenture Life Sciences Cloud(ALSC) - Clinical Operations Insights Platform(COIP) and our work with Metrics Champion Consortium(MCC) to answer the above questions.
8:30 Chairperson’s Remarks
Kuno van der Post, MSc, PhD, Chief Commercial Officer, OmniComm
8:35 Optimize Your Clinical Trials Using Electronic Health Records: The Case of EHR4CR
 Xia Wang, Ph.D., Director, Health Informatics, Global Medicines Development Unit, R&D, AstraZeneca
Xia Wang, Ph.D., Director, Health Informatics, Global Medicines Development Unit, R&D, AstraZeneca
This talk presents the AZ coordinated IMI project EHR4CR (Electronic Health Records for Clinical Research). The objective of EHR4CR is to research and develop a trustworthy technical platform and services to allow re-use of EHR data to
support clinical research in Europe. We will also share our experiences to test and evaluate the EHR4CR InSite platform for protocol feasibility and recruitment services on a growing hospital network.
9:00 Platform-Based Approaches in RWE: Approaches, Methodologies and Examples
 Jyotsna Mehta, MS, B.Pharm., Principal and Owner, KevaHealth
Jyotsna Mehta, MS, B.Pharm., Principal and Owner, KevaHealth
This presentation will provide an in-depth understanding of platform-based approaches in real world evidence and provide examples to show its usefulness and value in drug development throughout the life cycle of drug products. It will
also provide a framework of how and when to consider these for your studies and explain pros and cons through the use of examples.
9:25 Building Credibility with the Audience: Methodology
 Elizabeth MacLean, Pharm.D., Director, Global Health and Value/Outcomes & Evidence,
Pfizer
Elizabeth MacLean, Pharm.D., Director, Global Health and Value/Outcomes & Evidence,
Pfizer
Interest in the use of real world evidence to inform decision making in healthcare is growing. Importantly, concerns have been raised in the scientific and decision making communities regarding the reproducibility of observational
studies using large healthcare databases. This presentation will review the concepts of reproducibility and transparency and efforts to guide researchers in this regard.
9:50 Real World Evidence Based on Electronic Health Record (EHR) Data Requires Transparency in Key Operational Decisions
 Irene Cosmatos, Software Delivery Manager, Database Analytics Automation, UBC
Irene Cosmatos, Software Delivery Manager, Database Analytics Automation, UBC
Lack of transparency in research conducted using longitudinal healthcare databases has led to discrepancies in results and reduced confidence in evidence. Small differences in study definitions can impact study results and conclusions.
This presentation examines the impact of using different database algorithms for defining a patient’s activity period and length of continuous exposure definitions for various study outcomes when identifying a cohort of heart
failure patients to evaluate medication switching.
10:15 Networking Coffee Break
10:30 Chairperson’s Remarks
Kuno van der Post, MSc, PhD, Chief Commercial Officer, OmniComm
10:35 Enhancing Clinical Research with Data and Technology at an Academic Health System
 Sean Mooney, Ph.D., Chief Research Information Officer, UW Medicine
Sean Mooney, Ph.D., Chief Research Information Officer, UW Medicine
At the University of Washington, there are many clinical research touch points with our health system, UW Medicine. Not surprisingly, this is especially relevant in activities surrounding data and information technology. In this presentation,
I will discuss our efforts to leverage data and IT platforms to support research activities throughout the enterprise.
 11:00 Co-Presentation: Online Health Communities: A New Frontier in Clinical Research
11:00 Co-Presentation: Online Health Communities: A New Frontier in Clinical Research
 Olivier Chateau, CEO & Co-Founder, Health Union
Olivier Chateau, CEO & Co-Founder, Health Union
 Lauren Lawhon, COO, Health Union
Lauren Lawhon, COO, Health Union
Today's technology-equipped patient uses the Internet to communicate with their clinicians, connect with fellow patients, share their experiences, and track their own health. Millions of these engaged patients, sharing common ailments,
make up the rising group of online health communities. Members connect over common experiences, frustrations, and questions - and a wealth of insights can be gleaned from these online interactions. Learn how online health communities
provide valuable real-world evidence to advance clinical research.
11:25 pm Embedding Clinical Trials in the Electronic Medical Record: Challenges of Integrating Research into Clinical Care
Sarah Leatherman, Associate Director, Point of Care Program, Cooperative Studies Program Massachusetts Veterans Epidemiology Research & Information Center, Veterans Health Administration
Point-of-care clinical trials (POC-CT) are a pragmatic trial design intended to reduce the burden of research for both patient and provider and to support the learning healthcare system within the VA Healthcare System. Trials are fully
embedded in the electronic medical record and use only data that can be found in the corresponding data warehouse, Medicare, and the National Death Index. With this innovative design come a number of challenges associated with
regulation and implementation. We will discuss these challenges and their solutions in an active clinical trial.
11:50 Transition to Shared Sessions
12:00 pm CASE STUDY: The Art and Science of Patient Engagement in the Digital Era – Learning from GSK PARADE Study
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
The ability to efficiently develop new medicines for patients with unmet needs is limited by the current model for clinical development. Although the emerging mHealth technologies have the potential to improve the conduct of clinical
trials, the successful implementation requires careful study design and patient engagement. Data and experiences from GSK PARADE study will be summarized, highlighting the learning and opportunities in this new area of clinical
development.
12:25 Mobile Clinical Trials: New Findings on Patient and Site Perspectives from the Clinical Trials Transformation Initiative
Hassan Kadhim, Business Consultant for Clinical Operations, Boehringer Ingelheim Pharmaceuticals
This presentation will discuss qualitative and quantitative research conducted by the Clinical Trials Transformation Initiative to understand the perspectives of patients and site investigators of mobile clinical trials. Discussion
will include insights and advice from site investigators who have participated in trials that incorporate mobile devices to collect data for study endpoints.
12:50 PANEL DISCUSSION: Why Are There Barriers to the Adoption of Innovative Processes and Technologies at Sites?
Moderator: Jim Kremidas, Executive Director, Association of Clinical Research Professionals (ACRP)
Panelists: David Vulcano, Assistant Vice President & Responsible Executive for Clinical Research, Hospital Corporation of America (HCA)
Sean Walsh, MBA, CDO, Raleigh Neurology Associates
Beth Harper, MBA, Workforce Innovation Officer, Association of Clinical Research Professionals (ACRP)
Many innovative technologies and process improvement initiatives are coming out at a rapid pace, whether from TransCelerate and other industry consortia, or from technology companies themselves. Which of these improvements actually
work? How can sites implement these more effectively? Why are there barriers to adoption and how can the innovators better understand sites’ needs?
- Share sites’ perspective on the evolving clinical research landscape
- Discuss the reasons sites struggle with new processes and technology tools
- Determine ways to facilitate adoption
1:15 Closing Remarks
1:20 SCOPE Summit 2018 Adjourns
 Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple attendees
from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
 Marina Filshtinsky, M.D.
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1)
781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com