Cambridge Healthtech Institute’s 2nd Annual
Leveraging Real World Data for Clinical and Observational Research:
Integrating Evidence Generation with RWD
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
The abundance of data generated during routine health care is growing in significance and should be re-used for clinical and observational research. Patient electronic records, registries, insurance claims, data from pharmacy and social media, and electronic patient-reported outcomes have been increasingly used as eSources. This process requires strategizing, utilizing novel data technologies, as well as close collaboration between pharmaceutical companies and organizations that possess the data. CHI’s 2nd Annual “Leveraging Real World Data for Clinical and Observational Research” will discuss challenges and solutions with secondary use of existing healthcare data for assessing the effectiveness and safety of medical products.
Final Agenda
Wednesday, January 25
12:05 pm Bridging Luncheon Presentation: Leveraging Educational Materials in the Site and Patient Engagement for Observational Research
 Heather Gartman, Regional Managing Director, Public Relations Group, inVentiv Health
Heather Gartman, Regional Managing Director, Public Relations Group, inVentiv Health
 Julie Randolph, Ph.D., Project Director, Phase IV Operations, inVentiv Health
Julie Randolph, Ph.D., Project Director, Phase IV Operations, inVentiv Health
Researchers must be highly attuned to an increasingly engaged, well-informed, and metric savvy patient population. Developing meaningful research relationships with patients drives successful real-world evidence generation.
-Spark patient interest in observational research opportunities by demonstrating participation value
-Explore physician-patient connectivity strategies driving ongoing engagement
-Strengthen patient-site relationships via multiple points-of-contact
-Analyze patient feedback from recent clinical research experience
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes (see page 9 for details)
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairperson’s Remarks
Qin Ye, M.D., MS, Associate Principle, R&D Excellence, ZS Associates
4:05Co-Presentation: Systematic Approach to Use RWD to Inform Trial Design: Going beyond Simple Feasibility
 Hui Cao, M.D., Ph.D., Executive Director, Real-World Evidence for Respiratory, COE for RWE, Global Medical Affairs, Novartis Pharmaceuticals Corporation
Hui Cao, M.D., Ph.D., Executive Director, Real-World Evidence for Respiratory, COE for RWE, Global Medical Affairs, Novartis Pharmaceuticals Corporation
Qin Ye, M.D., MS, Associate Principle, R&D Excellence, ZS Associates
The use of Real World Data (RWD) to optimize trial design has been widely recognized. However, the majority of work in this area has been providing simply trial feasibility, i.e. patient counts based on the inclusion and exclusion criteria. We will present our ground-breaking pilot work that goes beyond the feasibility and applies a structured, systematic approach to assess the impact of each item in the trial criteria on recruitability, efficacy endpoints and risk. The combined insights will allow the trial team to design a clinical trial that could include most real-world patients without compromising the efficacy and increasing the potential risks.
 4:30 Impact of ICD-10 Transition on Conducting Retrospective Observational Database Studies and Pragmatic Clinical Trials
4:30 Impact of ICD-10 Transition on Conducting Retrospective Observational Database Studies and Pragmatic Clinical Trials
Rebecca Levin, MPH, Senior Research Scientist, UBC
U.S. healthcare providers are now required to use the ICD-10-CM version of the WHO’s disease classification which offers a substantial increase in the number and specificity of disease and procedure codes over ICD-9-CM. Our presentation will explain significant enhancements with ICD-10 and describe the strengths and limitations of available mapping tools to translate between ICD-9 and ICD-10 codes.
4:55 Empowered Patients + Electronic Health Records + Data Access = Transformational Opportunity for Research
 Craig Lipset, Head of Clinical Innovation, Pfizer
Craig Lipset, Head of Clinical Innovation, Pfizer
As a result of legislation in the United States, patients have a legal right to access their electronic health data in the format in which it is being maintained by their providers. According to multiple studies, when patients have such access the vast majority are willing to share that data to advance research (so long as their privacy wishes are maintained). When patients can bring their own EHR data into the research ecosystem, transformational opportunities emerge in patient recruitment, patient screening, and eSource data capture. The past year has seen milestones of progress toward this desired future. The White House Precision Medicine Initiative provided funding for Sync for Science, with commitments from the largest EHR vendors to support such patient-centered data movement for research. Even the most recent iOS update from Apple is creating enabling opportunities, as HealthKit can now enable consumers to load and share their EHR data. Enabling this future state is brings benefits for all stakeholders in the research ecosystem, from research sponsors to the patients looking to participate in finding new cures.
5:30INTERACTIVE PANEL: RWD to Inform Trial Design
Moderator: Jane Fang, M.D., Head, Research & Development Information and Analytics for Clinical Biologics, AstraZeneca/MedImmune
Panelists:
Kyle Flickinger, Vice President, Clinical Markets, HealthVerity
Qin Ye, M.D., MS, Associate Principle, R&D Excellence, ZS Associates

5:45 Reception hosted by Exostar
Thursday, January 26
7:15 am Registration
7:30 Co-Breakfast Presentation: The Inspiring Hope Ideathon: Solutioning the Clinical Trial Awareness Gap

Christine Phillips, Senior Director, Site & Patient Access, INC Research
 Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
To advance society’s ability to respond to future healthcare challenges and advance medical innovation we must increase awareness of clinical research and study participation. Clinical research is vital to the development of new drugs and treatments but is dependent on patient participation. The “Inspiring Hope Ideathon” was the first initiative of its kind designed to generate new and unique ideas. The participation and results were groundbreaking and will be shared here!
8:35 Chairperson’s Remarks
Jyotsna Mehta, MS, B.Pharm., Director, Economics Value Evidence and Outcomes, Alkermes, Inc.
8:40 FEATURED PRESENTATION: From Efficacy to Effectiveness: Studying the Effects of Medicines in Usual Care Settings
Andrew Roddam, Ph.D., Vice President & Head, Real World Evidence and Epidemiology, R&D Projects, Clinical Platforms & Sciences, GSK
This talk will discuss the opportunities available to study the effects of medicines in more usual care settings than is typical in controlled clinical trials. We will discuss the challenges encountered in trying to design and operationalise such studies as well as discussing the opportunities presented by the ability to utilise technologies such as EHRs and digital data capture to make the experience more real-life for patients and physicians.
9:05 Expanding Insight into Real World Oncology Practice through Linked Datasets
Elizabeth MacLean, Pharm.D., Director, Global Health and Value/Outcomes & Evidence, Pfizer
Prescription records alone provide limited information on patient characteristics and other treatment experience. However, linking datasets can broaden insight into patient, provider and reimbursement characteristics. This presentation will discuss the experience of linking a de-identified specialty pharmacy database with de-identified medical and pharmacy databases to examine real world use of axitinib in patients with renal cell carcinoma.
9:30 Leverage RWE Data in Clinical Trial Protocol Design and Site/PI Selection
 Jane Fang, M.D., Head, R&D Information and Analytics for Clinical Biologics, AstraZeneca/MedImmune
Jane Fang, M.D., Head, R&D Information and Analytics for Clinical Biologics, AstraZeneca/MedImmune
This presentation will provide use case examples on how to use real world evidence data and trial competition analytics to optimize clinical trial protocol development, patient population identification, patient recruitment and site/PI selection. The talk will also cover the strategy and business adoption to use RWE and trial competition information in today’s drug clinical pipeline development.
9:55 The "How" and "Why" of Leveraging Real World Data for Clinical and Observational Research
 Amy Ryan, M.S., Director of Biostatistics, Phase IV Operations, inVentiv Health
Amy Ryan, M.S., Director of Biostatistics, Phase IV Operations, inVentiv Health
Data from the real world shows what is actually going on in regular clinical practice. Some would say this data, often “messy”, and offers no real value in health outcomes research. This is not the case. The usefulness of the data is that it reflects our uncontrolled real world experiences. Knowing when to use this data is very important, but also knowing how to use this data is the true key to its value.
10:20 Coffee Break
10:35 Chairperson’s Remarks
Kelly Zou, Ph.D., PStat®, Senior Director and Analytic Science Lead, Real World Data & Analytics (RWDnA), Patient & Health Impact (P&HI), Pfizer, Inc.
10:40 Approaches and Methodologies to Develop High Quality Databases and Real World Evidence (RWE) Ecosystem for Observational Research
Jyotsna Mehta, MS, B.Pharm., Director, Economics Value Evidence and Outcomes, Alkermes, Inc.
Building a powerful platform of real-world data (RWD) is essential to providing leading-edge clinical, medical and commercial insights to the entire organization. However, developing meaningful and high quality databases warrants application of intricate data science that entails the ability to link detailed patient characteristics and information flows across different data sets to create a singular de identified architecture, and provide a complete 360 degree view of the health care ecosystem. This requires in-depth understanding of the data landscape, interface of different data variables of interest and touch points with the disease, patient population, and vertical & horizontal aspects of health care systems, along with application of enterprise level analytics. The presentation will focus on assessing different real world data sources, analytics and applications, and practical dimensions of developing & linking “fit for purpose” claims-EMR-Genomics-Lab linked data platforms to drive optimal study designs and high quality observational research.
 11:05 Applying the OMOP data model & OHDSI software to national European health data registries: the IMI EMIF project
11:05 Applying the OMOP data model & OHDSI software to national European health data registries: the IMI EMIF project
 Kees Van Bochove, MSc, CEO, The Hyve
Kees Van Bochove, MSc, CEO, The Hyve
A large open source initiative for standardisation and epidemiological analysis for real world data is OHDSI: Observational Health Data Sciences and Informatics. OHDSI leverages the OMOP common data model for observational data, and provides data analysis tools for a broad range of use cases. This talk will explain OMOP and OHDSI with case study IMI EMIF, in which health data from over 50 million patients from 13 national and regional European registries is brought together.
11:35Accessing and Generating Real-World Evidence via DataMart and Distributed Research Network
 Kelly Zou, Ph.D., PStat®, Senior Director and Analytic Science Lead, Real World Data & Analytics (RWDnA), Patient & Health Impact (P&HI), Pfizer, Inc.
Kelly Zou, Ph.D., PStat®, Senior Director and Analytic Science Lead, Real World Data & Analytics (RWDnA), Patient & Health Impact (P&HI), Pfizer, Inc.
A Real-World DataMart contains claims and transactions for healthcare resource utilization, electronic health records, surveys, linked datasets and other digital data collected outside a traditional clinical trial. To enhance the effectiveness and efficiency of health care delivery, it is important to understand risk factors for disease progression, treatment patterns, and utilization. Fruitful collaborative research opportunities exist across different healthcare stakeholders including academia, industry and government. A distributed research network is useful for generating real-world evidence. Examples on collaborative observational studies are illustrated.
Amstat News just published an interview with Dr. Zou titled “Kelly Zou: Mathematics, Statistics, Data Science, and Dreams.”
Wiley's StatisticsViews just named her as one of the "Most Inspirational Women in Statistics and Data Science."
12:00 pm Extracting Additional Value from Clinical Data
 Edward Bowen, Head, Data Science and Solutions, GSK
Edward Bowen, Head, Data Science and Solutions, GSK
TransCelerate is leading a collaboration across 12 companies to share placebo and standard-of-care (PSOC) clinical data for secondary research. Realized use cases include developing a standing safety cohort for providing context around SAEs observed in ongoing trials, and using data from prior trials to reduce the number of patients in new proof-of-concept trials. This discussion will discuss use cases for PSOC data, challenges around data sharing, successes to date, and important patient benefits.
12:25 Addressing the Critical Pieces in Utilizing Real World Patient Data, Key Success Factors of Evidence Generation
Kyle Flickinger, Vice President, Clinical Markets, HealthVerity
Tim McGarty, MBA, Global Category Manager, Digital Development, eCOA, PR&, Novartis
Dave Billiter, MBA, Director-Data Strategy & Product Development, Specialty Solutions, Cardinal Health
Qin Ye, M.D., MS, Associate Principle, R&D Excellence, ZS Associates
This presentation will focus on the key aspects of RWD evidence generation and how both clinical and observational can utilize the same patient population. In the absence of a persistent common patient identifier, data linkage, and data discovery, has become one of the rate limiting steps in both de-identifiable and identifiable RWE use cases. Early data linkage directly from the data source at the patient level can provide a more complete view of a patient’s medical information and allow researchers the transparency to target the specific patient population & data required for a RWD analytics.
12:50 Closing Remarks
12:55 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
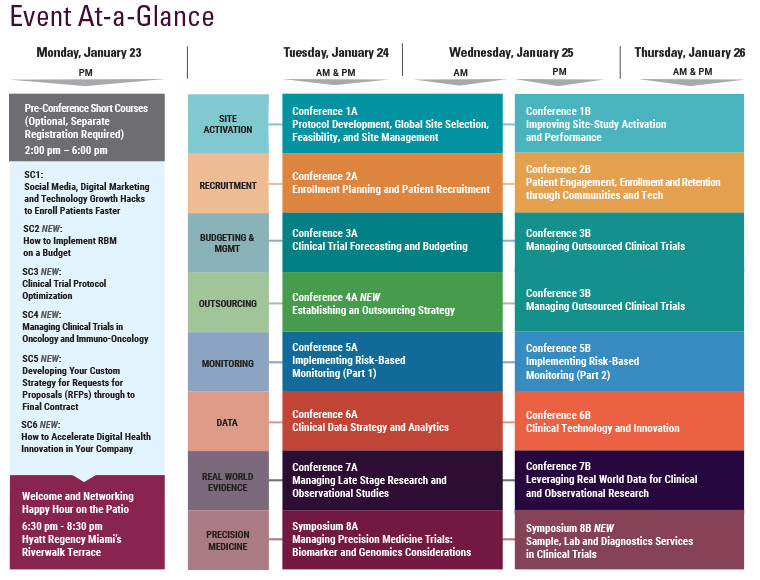
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Marina Filshtinsky, M.D.
Senior Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5496
E: mfilshtinsky@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com