Cambridge Healthtech Institute’s 4th Annual
Patient Engagement, Enrollment and Retention through Communities and Technology:
Patient Centric Approaches to Optimize Clinical Trial Participation
January 25-26, 2017 | Hyatt Regency Miami | Miami, FL
Enrollment planning and patient recruitment are critical to drug development programs and garner a lot of attention by study teams. However, once the hard work of identifying and recruiting a trial subject has been accomplished, they must be retained and remain in compliance. Retention of patients throughout the life of a clinical trial is essential in order to have complete data sets for your analysis and subsequent filings. There are strategies, tools and techniques such as social media platforms and mobile technology, empowered patient communities, and a more informed patient population that need to be understood and engaged. Cambridge Healthtech Institute’s 4th Annual “Patient Engagement, Enrollment and Retention through Communities and Technology” conference will cover the topics one should consider when planning and strategically implementing a patient engagement and a patient retention plan in the digital age.
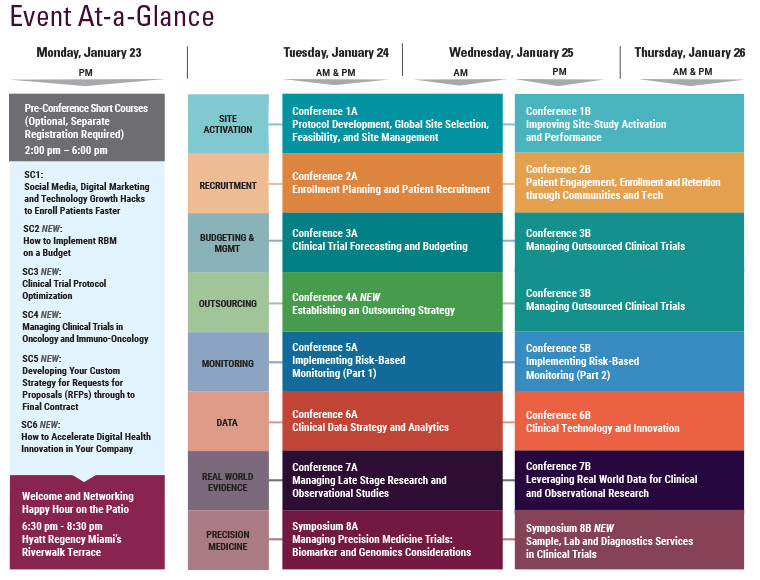
Final Agenda
Wednesday, January 25
 12:05 pm Bridging Luncheon Presentation: A Breakthrough in Technology Enabled Clinical Trials Recruitment
12:05 pm Bridging Luncheon Presentation: A Breakthrough in Technology Enabled Clinical Trials Recruitment
 Steven Coca, M.D., Associate Professor of Medicine, Internal Medicine, Icahn School of Medicine, Mount Sinai
Steven Coca, M.D., Associate Professor of Medicine, Internal Medicine, Icahn School of Medicine, Mount Sinai
Dr. Coca is presenting on the effectiveness of CLiX ENRICH in accelerating patient recruitment illustrated by two case studies: a retroactive analysis of a trial in which CLiX ENRICH yielded twice the candidates in half the time; and a live trial whereby CLiX identified a large cohort of quality patients.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Plenary Keynotes
3:00 Refreshment Break in the Exhibit Hall (Last Chance for Viewing)
4:00 Chairperson’s Remarks
Reg Blynn, Vice President, Client Services, Site and Patient Networks, QuintilesIMS
4:05 The Secret Weapon for Patient Engagement and Retention: Other Patients
 Roni Zeiger, M.D., Co-Founder, Smart Patients
Roni Zeiger, M.D., Co-Founder, Smart Patients
The traditional models define trial teams and investigators as experts who provide patients their knowledge and care. What if we redefine patients not just as partners, but also as valuable resources for each other before, during, and after trials? Doing so allows us to better support patients while engaging them as clinical trial participants and advocates.
4:30 Co-Presentation: Patient Engagement, Enrollment & Retention through Communities & Technology
 Lauren Johnson, Senior Recruitment Manager, Patient Access and Retention Services, PRA Health Sciences
Lauren Johnson, Senior Recruitment Manager, Patient Access and Retention Services, PRA Health Sciences
 Gretchen Goller, MSW, Senior Director PARS, PRA Health Sciences
Gretchen Goller, MSW, Senior Director PARS, PRA Health Sciences
 Susan Campbell, Director, Patient Access and Retention Services, PRA Health Sciences
Susan Campbell, Director, Patient Access and Retention Services, PRA Health Sciences
There is a growing consensus with industry experts and stakeholders regarding the importance of understanding the patients’ needs and experiences to drive clinical trial design and ensure patients stay engaged and compliant throughout their participation. Patient feasibility is an area underexplored in the clinical trial industry. Although protocol and site feasibility services have become standard activities of sponsors and CROs in order to plan and operate clinical trials, patient feasibility has currently not been adopted in the same way. This is partially due to the lack of knowledge of this service offering. Patient feasibility can yield quantitative and qualitative data that offers sponsors insight into patients’ motivations and intentions in participating in clinical trials. PRA’s strategy is aimed at understanding patient’s decision-making process and perceptions about clinical trial participation and protocol-specific studies in order to preemptively identify potential enrollment and retention barriers and key messaging from the patient’s perspective. We will be discussing several case studies in this session that demonstrate the importance of this deeper dive into the patient perspective and illustrating that feedback to inform all aspects of a patient recruitment campaign.
4:55 CO-PRESENTATION: Inside Out: A New Approach to Patient Engagement
 Helen Kellar-Wood, Ph.D., Lead, Immunoscience Diversity & Patient Engagement, Global Clinical Operations, Bristol-Myers Squibb
Helen Kellar-Wood, Ph.D., Lead, Immunoscience Diversity & Patient Engagement, Global Clinical Operations, Bristol-Myers Squibb
 Mary Murray, Associate Director, Diversity & Patient Engagement, Global Clinical Operations, Bristol-Myers Squibb
Mary Murray, Associate Director, Diversity & Patient Engagement, Global Clinical Operations, Bristol-Myers Squibb
Patient engagement does not always look and feel the same from internal and external perspectives. How can patient engagement strategies be developed to perpetuate an ongoing and robust conversation with patients as partners? The speakers will address this question, sharing examples and unexpected experiences from the past few years that have led to the development of a new approach.
5:20 CO-PRESENTATION: Patient Group Engagement in Clinical Trials: Best Practices for Best Value
 David Leventhal, Director, Clinical Innovation, Worldwide R&D, Pfizer, Inc.
David Leventhal, Director, Clinical Innovation, Worldwide R&D, Pfizer, Inc.
 Ken Getz, MBA, Director, Sponsored Research Programs, Tufts CSDD; Chairman, CISCRP
Ken Getz, MBA, Director, Sponsored Research Programs, Tufts CSDD; Chairman, CISCRP
Patient groups are developing diverse skillsets and assets to provide valuable trial services, funding, and ability to enhance collaboration. Research sponsors across the clinical trials enterprise are recognizing the benefits of continuous and meaningful patient group engagement, but all stakeholders need further guidance on operationalizing this new model. CTTI’s best practices consolidate actionable recommendations for establishing strong, active patient group engagement during all phases of the research and development lifecycle.
 5:45 Reception hosted by Exostar
5:45 Reception hosted by Exostar
Download Brochure
Thursday, January 26
7:15 am Registration
7:30 Co-Breakfast Presentation: The Inspiring Hope Ideathon: Solutioning the Clinical Trial Awareness Gap
 Christine Phillips, Senior Director, Site & Patient Access, INC Research
Christine Phillips, Senior Director, Site & Patient Access, INC Research
 Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
Angela Radcliffe, Executive Vice President, Senior Leadership, FCBVIO
To advance society’s ability to respond to future healthcare challenges and advance medical innovation we must increase awareness of clinical research and study participation. Clinical research is vital to the development of new drugs and treatments but is dependent on patient participation. The “Inspiring Hope Ideathon” was the first initiative of its kind designed to generate new and unique ideas. The participation and results were groundbreaking and will be shared here!
8:35 Chairperson’s Remarks
Aaron Fleishman, Product Innovation and Engagement Strategist, Market Expansion and Product Innovation, BBK Worldwide
8:40 The Hero’s Journey: Crowdsourced Art to Celebrate Clinical Trial Participants and Raise Awareness of Clinical Research
 Kelly McKee, Advisor, Clinical Innovation, Eli Lilly and Co.
Kelly McKee, Advisor, Clinical Innovation, Eli Lilly and Co.
The Hero’s Journey is crowdsourced art sponsored by Lilly to celebrate clinical trial participants and raise awareness of clinical research. Once complete, the sculptures will be displayed to the public as part of an art exhibit traveling throughout the United States and will be on permanent public display after the traveling exhibit ends. We will engage communities and raise awareness of clinical trials through the artwork and social media using #herosjourneyart. The project will be complete with results to share in time for SCOPE 2017.
9:05 Improving the Clinical Trial Patient Experience by Effectively Engaging Patients
 Michelle Collins, Director, Patient and Investigator Relations, Clinical Field Operations, AbbVie
Michelle Collins, Director, Patient and Investigator Relations, Clinical Field Operations, AbbVie
Enabling timely patient recruitment and maintaining robust patient retention continue to be key challenges in the conduct of clinical trials. Effective patient engagement early in the clinical development process can significantly improve patient recruitment and retention efforts. Additionally, effective patient engagement can also inform clinical trials to improve the experience of patients participating in clinical trials. Enhancing the patient experience is a key objective that should be considered when implementing patient engagement efforts in clinical development.
9:30 Informed Consent Entering the Digital World: The Transcelerate eConsent Initiative
 Holly Beisner, Senior Associate, Site Activation Management, Clinical Operations, Eli Lilly and Company
Holly Beisner, Senior Associate, Site Activation Management, Clinical Operations, Eli Lilly and Company
Co-developed with Hilde Vanaken, Ph.D., Director, R&D Operations Innovation, Janssen Research & Development
While the shift to digital technologies is pervasive, the informed consent process for clinical trials remains paper-based. eConsent can transform the informed consent process by using an array of patient-focused digital components to empower patients and their caregivers to make an informed decision and create process efficiencies for sites, health authorities, IECs/IRBs and sponsors. The eConsent Initiative at TransCelerate provides the first cross-industry perspective on this novel technology, developed over a period of 1.5 years with input from over 14 global pharmaceutical companies.
9:55 Patient Recruitment of Alzheimer’s Disease: Using Behavioral Research to Create Lasting Engagement and Trial Success
 Barbara Zupancic, MS, MBA, Director, Global Patient Recruitment and Retention, Worldwide Clinical Trials
Barbara Zupancic, MS, MBA, Director, Global Patient Recruitment and Retention, Worldwide Clinical Trials
Alzheimer's research is booming and many believe the first person cured from this disease has already been born. Conducting studies in people who exhibit no cognitive deficits (yet) will be the differentiator in finding a cure.
Audiences can expect to learn how to effectively conduct and use findings from Worldwide’s extensive Alzheimer's experience. Attendees will learn best practices for developing/implementing a patient-centered strategy focused on using the right channels/messaging for lasting engagement and faster recruitment.
10:20 Coffee Break
10:35 Chairperson’s Remarks
Matt Hendricks, Partner, Pharmica Consulting
10:40 Content Really Is King! Even in Clinical Research
 Jerry Matczak, Consultant, Clinical Innovation, Eli Lilly and Company
Jerry Matczak, Consultant, Clinical Innovation, Eli Lilly and Company
Fresh, engaging and ultimately authentic, human content is required to connect to people in today’s information saturated and very social world. Clinical research is no exception. Learn from Lilly’s experience in bringing a content marketing strategy to clinical research through a multi-channel, internet engaged ecosystem to raise awareness, trust and ultimately participation in clinical research.
11:05 Multi-Channel, Multi-Platform for Improved Patient Engagement
 Aaron Fleishman, Product Innovation and Engagement Strategist, Market Expansion and Product Innovation, BBK Worldwide
Aaron Fleishman, Product Innovation and Engagement Strategist, Market Expansion and Product Innovation, BBK Worldwide
Approaches that include new innovations more often than not see improved engagement, more reliable data and higher retention rates. This session will take a look at how to implement innovation company-wide. We will look at the most disruptive innovations and offer ideas on how to implement them to the benefit of patients and study stakeholders. Also, practical advice on preparing for what’s next in the industry, building innovation, and growing with new technology.
11:30 Transition to Shared Sessions
11:35 Chairperson
Matt Hendricks, Partner, Pharmica Consulting
11:40 Remote Trials: Moving beyond the Concept
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer Ingelheim
Remote Trials have been gaining more traction over the past few years as a new and innovative way to run clinical trials. The concept is certainly very interesting, but operationally very challenging to coalesce. In this talk, we will address some of these challenges, review the stakeholders’ perceptions around the implementation of Remote Trials, and propose the steps forward to be able to run Remote Trials in the near future.
12:05 pm CoLAB: Redefining Collaborative Engagement with Patients in Clinical Trials
 Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
Megan Laker, CoLAB Consultant, CDIO, Eli Lilly and Company
The purpose of CoLAB is to improve Lilly clinical trials by considering the Site, Patient, and Patient-partner perspective. Site and Patient Simulation is one of the ways that CoLAB brings together Lilly study teams, clinical site Study Coordinators, Patients, and Patient-partners to understand real-world feedback on operational issues within our clinical protocols. By engaging your Patients upfront, you can ensure that good science aligns with good patient care. By engaging Patients early in protocol development, you can potentially improve the clinical research patient experience.
12:25 CO-PRESENTATION: Engaging with Sites and Patients to Enable Digital Innovation for Clinical Trials
 Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
Elizabeth Beatty, Head, Digital Clinical Trials, Bristol-Myers Squibb
 Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
Scott Rauscher, Associate Director, Global Procurement R&D, Bristol-Myers Squibb
In the new healthcare ecosystem and digital age, patients expect care and solutions that are coordinated, convenient, customized, and accessible. Biopharmaceutical companies are doing a lot to address these emerging expectations for patient engagement services and we are all learning a lot on the way. It is important to truly engage with sites, investigators and research volunteers using both traditional and hi-tech means and to learn from those early and ongoing interactions. With Aspire, a unique BMS effort that will be shared in this presentation, we put the focus on the Sites and Patients and the results are guiding other trial planning and management efforts.
12:55 INTERACTIVE PANEL: Digital Clinical Trial Lessons Learned: Panel Discussion from Pharma Innovators Who Have Run Virtual Trials
Moderator:
 Matt Hendricks, Partner, Pharmica Consulting
Matt Hendricks, Partner, Pharmica Consulting
 Hassan Kadhim, Business Consultant, IT RDM, Boehringer-Ingelheim
Hassan Kadhim, Business Consultant, IT RDM, Boehringer-Ingelheim
 Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
Michelle Crouthamel, Lead, Clinical Innovation & Digital Platforms Unit, GlaxoSmithKline
 Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
Alex Simmonds, Associate Director, Health IT, Bristol-Myers Squibb
 Margaretta Nyilas, MD, Sr. Vice President, Clinical and Business Operations
Margaretta Nyilas, MD, Sr. Vice President, Clinical and Business Operations
In the past year, several large Pharma companies have begun experimenting with a new breed of reimagined clinical trials which leverage wearables and fewer sites. Now the results from the first round of these experiments are in, and the pioneers who ran the studies are ready to share their findings. Join us as we discuss what aspects of these studies are ready for prime time, where there is still work to be done, and most importantly, how patients have reacted to this shift. The conversation will focus on platforms & technology from industry veterans, startups, and established newcomers such as Apple and their ResearchKit platform.
1:20 Closing Remarks
1:25 SCOPE 2017 Conference Adjourns (see you in Orlando for 2018!)
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com