Cambridge Healthtech Institute’s 7th Annual
Clinical Trial Forecasting and Budgeting:
Innovative Budgeting and Contracting for Cost-Efficient Trials
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
The need for accurate trial forecasting, budgeting, and contracting is continuing to grow as costs continue to rise and pressure to do more with less increases. Developing strategies for better financial planning, budgeting, and contracting can reduce these pressures and lead to streamlined, cost-efficient trials. Cambridge Healthtech Institute’s Seventh Annual "Clinical Trial Forecasting and Budgeting" conference shares best practices and case studies on building more effective budgets and contracts as well as innovative strategies in communicating and negotiating costs with vendors and CROs.
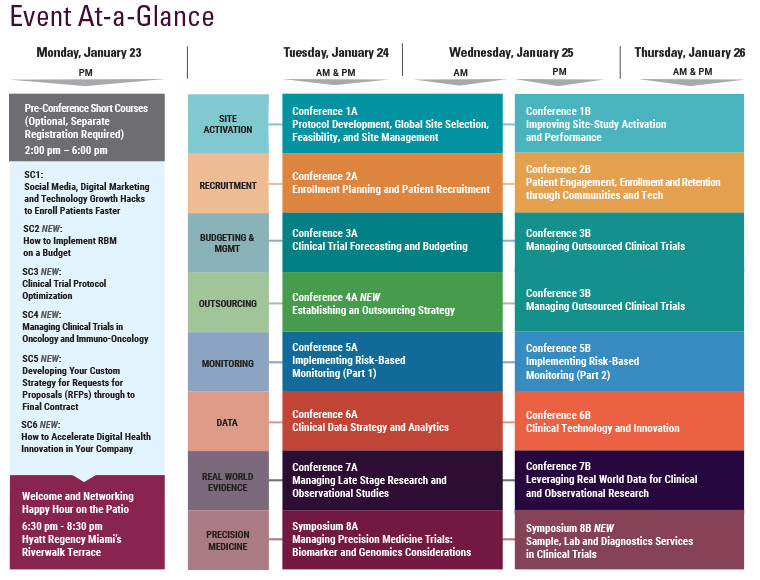
Final Agenda
Monday, January 23
1:00 pm Short Course Registration
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations and Compliance, Boston University
10:50 Common Pitfalls Associated with Budgeting and Forecasting for Clinical Trials and How to Overcome Them
 Chris Chan, Senior Director, R&D Finance, Finance, FibroGen, Inc.
Chris Chan, Senior Director, R&D Finance, Finance, FibroGen, Inc.
There are pitfalls associated with budgeting and forecasting for clinical trials that a majority of companies experience, regardless of their size. This presentation will discuss some of these common pitfalls and ways companies can alleviate and overcome them.
11:15 Financial Forecasting for an In-House Clinical Trial
 Maryanne Santilli, Associate Director, Clinical Trial Management Finance & Operations, Novo Nordisk
Maryanne Santilli, Associate Director, Clinical Trial Management Finance & Operations, Novo Nordisk
The level of granularity needed for forecasting an in-house clinical trial is great. The sponsor must negotiate with investigators, assess fair market value, and account for lab and ad hoc costs. This talk will examine Novo Nordisk’s strategy for financial forecasting as well as discuss tips for working with investigators.
11:40 Sponsor Strategies for Improved Investigator Site Payments
 Débora Araujo, Associate Director, Clinical Contracting Services, Boehringer Ingelheim Pharmaceuticals, Inc.
Débora Araujo, Associate Director, Clinical Contracting Services, Boehringer Ingelheim Pharmaceuticals, Inc.
As the years pass and many aspects of clinical trials are improved, many of the same concerns can be heard from clinical investigative sites regarding sponsor payments. Regardless of the size of the sponsor or systems available, there are some strategies and practical tips which, if implemented, can help streamline and improve the sponsor-site relationship in the area of investigator site payments.
12:05 pm A View into the Future of Payment Technology
Lori McClain, Vice President, Product Management, Bioclinica
Site and patient payments are an important part of clinical trials, and a vital data source for budgeting and forecasting. The new wave of innovation in this space is driving insight into both expense and resource forecasting, even financial management for sites. This session will focus on ways to harness this innovation and run trials that are more attractive to sites, more engaging for patients, and less of a financial burden on the sponsor.
 12:40 Luncheon Co-Presentation: Leveraging Improved Data Quality and Data Aggregation for Greater Vendor Analysis and Insight
12:40 Luncheon Co-Presentation: Leveraging Improved Data Quality and Data Aggregation for Greater Vendor Analysis and Insight
 Lior Keet, Vice President, Life Sciences Research & Development, HighPoint Solutions
Lior Keet, Vice President, Life Sciences Research & Development, HighPoint Solutions
 Jennifer Gilletti, Executive Director, Sourcing Operations Head, Development Operations, Pfizer, Inc.
Jennifer Gilletti, Executive Director, Sourcing Operations Head, Development Operations, Pfizer, Inc.
Life Sciences organizations can miss opportunities relative to securing a fair and competitive price with vendor partners. Sponsors must be able to effectively and efficiently manage and analyze a vast amount of information, including detailed activity based budgets and their associated assumptions, as part of the bid review process in order to identify the appropriate vendor/s to support the given clinical study program. However, the traditional vendor proposal process generates heterogeneous data that negatively impacts the Sponsor's ability to conduct a true vendor comparative analysis.
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Débora Araujo, Associate Director, Clinical Contracting Services, Boehringer Ingelheim Pharmaceuticals, Inc.
2:05 CO-PRESENTATION: Modernize Clinical Investigator Payment Process with New Technologies and Integrations
Andy Chung, Sr. Manager Information Systems, Clinical Trial Design and Management, Amgen
Iain Wood, Clinical Pricing Manager, Amgen
Inefficiencies and delays in Clinical Site Payment process remains one of the biggest pain points for both - Clinical Sites and Sponsors. This presentation will share an integrated approach to leverage new technologies and clinical data flow for optimizing payment process for all parties involved. Discussion will include lessons learned from a global implementation.
2:30 PANEL DISCUSSION: Negotiating Site Budgets: When Enough is Enough
Moderator:
 Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Panelists:
 JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
 Janis Witzleb, Head, Study Operations, CSL Behring
Janis Witzleb, Head, Study Operations, CSL Behring
Kimberly Ray, Vice President, Site and Patient Networks, Quintiles IMS
This panel discussion will raise questions to the audience about experience negotiating site budgets between the CRO, Sponsor, and site, the types of issues that arise, and the game of give and take. What is the break-even point for study sites? When do negotiations move forward and when do CROs, sites, or sponsors walk away? We all face difficult negotiations, and often reach stalemate... Who gives in, and why?
2:55 Budgeting Globally with Sites for Rare Disease Clinical Trials
 Janis Witzleb, Head, Study Operations, CSL Behring
Janis Witzleb, Head, Study Operations, CSL Behring
For rare disease clinical trials, finding the right patients is a challenge – they could be anywhere in the world. This talk will discuss working with sites, choosing single versus multiple vendors, and ultimately strategies for budgeting with these sites. We will discuss discovering what the sites need and balancing local law in order to properly budget for your trial.
 3:20 Cost Drivers in Ancillary/Clinical Supply Chain
3:20 Cost Drivers in Ancillary/Clinical Supply Chain
 Peter Knapp, Vice President, Logistics and Global Expansion, Ancillare, LP
Peter Knapp, Vice President, Logistics and Global Expansion, Ancillare, LP
This session will focus on frequently neglected clinical trial forecasting and budgeting for ancillary (non-drug) clinical trial supplies for global studies. These supplies include, but not limited to, patient and site consumables, printed materials, nutritional products and equipment (freezer, centrifuges, etc.). Frequently, ancillary supplies represent significant cost, risk, and time drivers in global clinical trials. Topics will include: Techniques for the mitigation of financial risks through proper planning and forecasting, inventory management, plus more.
3:45 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, January 25
7:15 am Registration
7:30 Breakfast Presentation: eConsent: Put "Informed" Back in Informed Patient Consent
 Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Electronic informed consent makes the consenting process more efficient and effective for staff, sponsors, monitors, and most importantly patients by presenting the information in formats and language in which patients are comfortable. Join us for breakfast to learn the best practices, latest technological advances, and proven benefits of deploying an eConsent solution - including the impact it has on improving patient satisfaction and retention and help us put "informed" back in patient consent process.
8:25 Chairperson’s Remarks
Kym Denny, CEO, hVIVO
8:30 A Practical Approach to Managing the Change Order Process and Limiting the Number of Changes in Scope
 Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
This paper will discuss Pfizer’s approach to managing ongoing changes in scope including, but not limited to, identification of out-of-scope items, estimating cost, seeking approvals, and when to move to a contractual change in scope. Pfizer’s approach is rather complex, but boiling the process down to simple steps, defining the key players, and responsibilities in an ownership matrix, is key to following and successfully executed well-thought-out and valid changes in scope.
8:55 Contract Language and Limitations: A Site's Perspective
 JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
Clinical trial agreements (CTAs) are provided to the study site by sponsor or CRO as a contract template. Not all language terms in the CTA may apply to the site or a specific study and if left in the CTA can lead to a breach of contract. This session looks at the typical legal language contained in the CTA that can be problematic and what it means. We will compare examples of contract language and how to revise it in terms that are understandable and protect the site.
9:20 Challenges with Contracting for Investigator Sites and CROs: An Operational Perspective
 Bruno Gagnon, President, Xenon Clinical Consulting
Bruno Gagnon, President, Xenon Clinical Consulting
With the number of people involved as well as the sheer amount of factors to take into consideration contracting can become one of the biggest reasons why a clinical trial is delayed. This talk will address operational aspects of contracts and budgets for investigator sites, putting an emphasis on rapid start-up and how to pay for good performance, therefore, increasing quality and productivity.
9:45 Overseeing International Budgets and Contracts: The Importance of Cultural Awareness in Smooth Negotiations
 Kym Denny, CEO, hVIVO
Kym Denny, CEO, hVIVO
Study set up activities across multiple countries and sites is at best a complex juggling act. What often slows us down is a lack of practical local knowledge about the way people engage in relationships, negotiations, and how various healthcare structures translate into budgeted tasks. To succeed in this setting, it is important to have the right set of expectations and a solid understanding of how culture informs the way we approach collaborating on a project. The aim of this session is to explore how different cultural orientations impact on budget and contract negotiations, and to provide a framework for the global clinical project manager and lead CRA to operate confidently when working on international clinical studies.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
John Boland, Vice President, Product Development, Atlantic Research Group, Inc.
11:15 Panel Discussion: Communication for Strategic Partnerships: Establishing Relationships to Enable Better Negotiating and Contracting
Moderator:
Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations and Compliance, Boston University
Panelists:
Jay Zinni, Associate Director, Procurement, Incyte Pharmaceuticals
Luke Van Hengel, Corporate Vice President, Business Operations, PAREXEL International
Greg Skalicky, Chief Enterprise Business Officer, inVentiv Health
Relationships matter. This panel discussion will discuss strategies for establishing relationships between sponsors, CROs, and other suppliers and vendors. Panelists will explore ways establishing a solid relationship will enable better negotiating, contracting, and forecasting.
12:05 pm Bridging Luncheon Presentation: Introducing DrugDev Spark™ - Technology to Transform Clinical Operations
 Brett Kleger, Chief Commercial Officer, DrugDev
Brett Kleger, Chief Commercial Officer, DrugDev
DrugDev Spark™ is revolutionary clinical technology that brings all administrative solutions sponsors, CROs and sites need to run a trial together into one unified solution suite with a single sign on. Featuring solutions from site selection and activation, to payments, training and eConsent, all tied together with the DrugDev Golden Number DrugDev Spark is primed to transform the way global clinical trials are run. Join us for lunch and an exclusive preview at SCOPE!
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com