Cambridge Healthtech Institute’s Inaugural
Establishing an Outsourcing Strategy:
Identifying and Evaluating Third-Party Vendors and Partners
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
Before any clinical trial can begin, the needs of the trial need to be determined, and with the ever-growing need for efficient and cost-effective trials, establishing an outsourcing strategy for your company and for individual trials is key to keeping things on time and on budget. Cambridge Healthtech Institute’s Inaugural "Establishing an Outsourcing Strategy" conference shares strategies and case studies for determining outsourcing needs, developing RFPs and evaluating bids, as well as contracting with outsourced partners and vendors, including sites, CROs, suppliers, and other vendors.
Final Agenda
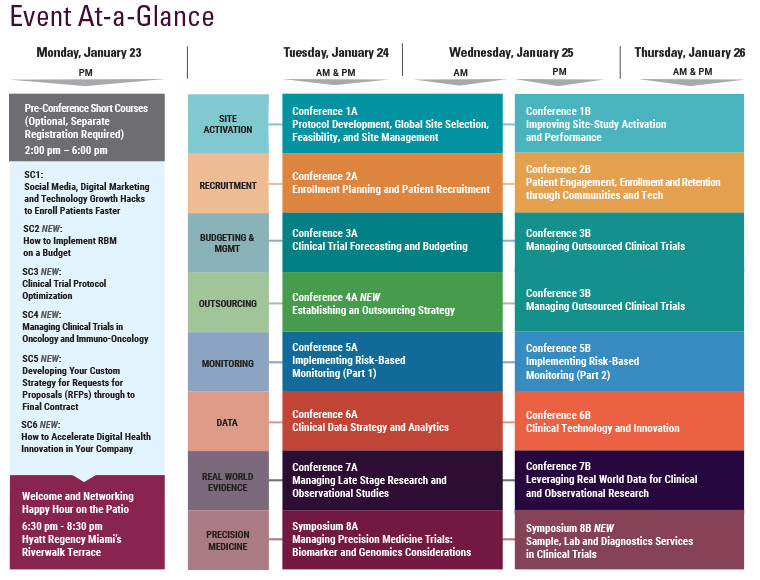
Monday, January 23
1:00 pm Short Course Registration
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
MaryAnne Rizk, Ph.D., Vice President, Global CRO and Biopharma Partnerships, Oracle Health Sciences
10:50 Establishing a Sourcing Strategy in Early Development
 Rich Polgar, Director, Global Procurement, Bristol-Myers Squibb
Rich Polgar, Director, Global Procurement, Bristol-Myers Squibb
This presentation will discuss how Bristol-Myers Squibb is challenging the role of the procurement department in its overall outsourcing strategy. This will be a case example of executing a relationship plan and business partnering to gather business needs, leverage market data, and develop an innovative strategy for implementing speed with the appropriate rigor in the process.
11:15 Strategies for Outsourcing a Large Development Portfolio
 Ratan Ratnesh, Director, Head, Clinical Outsourcing, Otsuka
Ratan Ratnesh, Director, Head, Clinical Outsourcing, Otsuka
This talk will cover how to develop clinical outsourcing strategy to support a large development portfolio. We will discuss developing category strategy to support the outsourcing requirements of your company, the assessment of internal operations and external market to support outsourcing decisions (make vs. buy, portfolio analysis, etc.), how to develop study level outsourcing decisions within the category level sourcing strategies, and supporting new innovation within the outsourcing framework. Finally, we will explore total value management as part of clinical outsourcing rather than focus on just the cost/savings.
11:40 Best Practices in Developing and Maintaining an Effective Clinical Outsourcing Strategy
 Todd Reul, Associate Director, Global Strategic Sourcing, BioMarin
Todd Reul, Associate Director, Global Strategic Sourcing, BioMarin
This talk will highlight ways to develop and maintain a global outsourcing strategy for your clinical pipeline. We will discuss best practices in regards to aligning with your company’s clinical development strategy, incorporating the subtleties of therapeutic areas, techniques to ensure outsourcing processes never limit operational progress, as well as applying lessons learned to continually improve and refine.
 12:05 pm ICH E6 Revision2: What Impacts Might This Have on an Outsourcing Strategy?
12:05 pm ICH E6 Revision2: What Impacts Might This Have on an Outsourcing Strategy?
 Nicole Stansbury, Executive Director, Clinical Management, PPD
Nicole Stansbury, Executive Director, Clinical Management, PPD
ICH E6 R2 suggests centralized monitoring be incorporated as an important monitoring methodology in clinical trials. Different companies are taking different approaches on deploying the centralized monitoring and these differences will require more detailed discussions when outsourcing clinical trial services. This presentation will cover some of the types of information that should be considered in outsourcing decisions, including additional details that may need to be specified in RFPs.
12:35 Session Break
12:40 Luncheon Presentation (Sponsorship Opportunity Available) or Lunch on Your Own
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Rich Polgar, Director, Global Procurement, Bristol-Myers Squibb
2:05 Bid Defense Tactics that Work
 Mark J. Milberg, Director, Clinical Procurement and Outsourcing, Ultragenyx Pharmaceutical Inc.
Mark J. Milberg, Director, Clinical Procurement and Outsourcing, Ultragenyx Pharmaceutical Inc.
In this presentation we will uncover the best practical applications of the bid defense meeting in clinical research. This is a critical portion of the overall bid evaluation and vendor selection process. There truly is not one standard way to reach your goals in deciding how it should be structured, who should participate, and what to focus on. We will also explore and learn together from collective past experiences where assumptions have tripped up success.
2:30 Driving Value Through RFPs – A Customized Approach to Developing RFPs with Internal Stakeholders
Benjamin Greenberg, Senior Manager, Lead, Clinical Outsourcing & Operational Analysis, Curis
In the fast-paced and demanding environment of clinical development, speed and quality are paramount, and selecting the right clinical vendor can be the difference between success and failure. By enabling key stakeholders to drive the value in RFP’s; the selection, quality, and time to implementation are all improved. The presentation will focus on a process and tools which empower stakeholders to drive a better vendor selection process and result.
2:55 Sourcing Need to Provider Selection Part I: A Strategy for Sourcing Initiatives and Developing Contracts
>Marija Nikolic, Associate Director, Development Operations, Contracts & Outsourcing, Vendor Management, Astellas Pharma Global Development, Inc.
Your company needs to select a new provider for a particular service. Whether to replace current providers or as a shift in sourcing strategy, we all face the same challenges. What’s the best process for identifying an appropriate provider pool? How to conduct a meaningful RFI process to narrow the pool? How to leverage the RFP process to optimize price for quality of service? These and others are questions we should be asking ourselves as part of this process. This talk will provide an overview of one strategy to successfully navigate through these tough discussions and negotiations.
 3:20 Is Your Data Platform Providing You Trust and Transparency in Your Outsourcing Partnerships?
3:20 Is Your Data Platform Providing You Trust and Transparency in Your Outsourcing Partnerships?
 MaryAnne Rizk, Ph.D., Vice President, Global CRO and Biopharma Partnerships, Oracle Health Sciences
MaryAnne Rizk, Ph.D., Vice President, Global CRO and Biopharma Partnerships, Oracle Health Sciences
The most successful, differentiated, outsourcing services depend on transparent, on demand data to build and construct working partnerships, ensuring trust based on measurable, quantifiable access and data visibility. This presentation will highlight how strategic partnerships go beyond CRO selection by jointly defining the ‘source of truth’ with comprehensive technology solutions focused around Trial Planning, Trial Management, and Trial Close. Outcomes include streamlined clinical programs, reduced trial costs and bringing drugs to market safer and faster.
3:45 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, January 25
7:15 am Registration
7:30 Breakfast Presentation: eConsent: Put "Informed" Back in Informed Patient Consent
 Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Electronic informed consent makes the consenting process more efficient and effective for staff, sponsors, monitors, and most importantly patients by presenting the information in formats and language in which patients are comfortable. Join us for breakfast to learn the best practices, latest technological advances, and proven benefits of deploying an eConsent solution - including the impact it has on improving patient satisfaction and retention and help us put "informed" back in patient consent process.
8:25 Chairperson’s Remarks
Kym Denny, CEO, hVIVO
8:30 A Practical Approach to Managing the Change Order Process and Limiting the Number of Changes in Scope
 Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
Kenneth Wilson, Director, Business Operations; Clinical Outsourcing Lead, Pfizer
This paper will discuss Pfizer’s approach to managing ongoing changes in scope including, but not limited to, identification of out-of-scope items, estimating cost, seeking approvals, and when to move to a contractual change in scope. Pfizer’s approach is rather complex, but boiling the process down to simple steps, defining the key players, and responsibilities in an ownership matrix, is key to following and successfully executed well-thought-out and valid changes in scope.
8:55 Contract Language and Limitations: A Site's Perspective
 JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
JoAnn Pfeiffer, DrSC., Director, Clinical Research Management, Arizona State University
Clinical trial agreements (CTAs) are provided to the study site by sponsor or CRO as a contract template. Not all language terms in the CTA may apply to the site or a specific study and if left in the CTA can lead to a breach of contract. This session looks at the typical legal language contained in the CTA that can be problematic and what it means. We will compare examples of contract language and how to revise it in terms that are understandable and protect the site.
9:20 Challenges with Contracting for Investigator Sites and CROs: An Operational Perspective
 Bruno Gagnon, President, Xenon Clinical Consulting
Bruno Gagnon, President, Xenon Clinical Consulting
With the number of people involved as well as the sheer amount of factors to take into consideration contracting can become one of the biggest reasons why a clinical trial is delayed. This talk will address operational aspects of contracts and budgets for investigator sites, putting an emphasis on rapid start-up and how to pay for good performance, therefore, increasing quality and productivity.
 Kym Denny, CEO, hVIVO
Kym Denny, CEO, hVIVO
Study set up activities across multiple countries and sites is at best a complex juggling act. What often slows us down is a lack of practical local knowledge about the way people engage in relationships, negotiations, and how various healthcare structures translate into budgeted tasks. To succeed in this setting, it is important to have the right set of expectations and a solid understanding of how culture informs the way we approach collaborating on a project. The aim of this session is to explore how different cultural orientations impact on budget and contract negotiations, and to provide a framework for the global clinical project manager and lead CRA to operate confidently when working on international clinical studies.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
John Boland, Vice President, Product Development, Atlantic Research Group, Inc.
11:15 Panel Discussion: Communication for Strategic Partnerships: Establishing Relationships to Enable Better Negotiating and Contracting
Moderator:
 Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations and Compliance, Boston University
Marina Malikova, Ph.D., MA, Executive Director, Surgical Translational Research Operations and Compliance, Boston University
Panelists:
Jay Zinni, Associate Director, Procurement, Incyte Pharmaceuticals
Luke Van Hengel, Corporate Vice President, Business Operations, PAREXEL International
 Greg Skalicky, Chief Enterprise Business Officer, inVentiv Health
Greg Skalicky, Chief Enterprise Business Officer, inVentiv Health
Relationships matter. This panel discussion will discuss strategies for establishing relationships between sponsors, CROs, and other suppliers and vendors. Panelists will explore ways establishing a solid relationship will enable better negotiating, contracting, and forecasting.
12:05 pm Bridging Luncheon Presentation: Introducing DrugDev Spark™ - Technology to Transform Clinical Operations
 Brett Kleger, Chief Commercial Officer, DrugDev
Brett Kleger, Chief Commercial Officer, DrugDev
DrugDev Spark™ is revolutionary clinical technology that brings all administrative solutions sponsors, CROs and sites need to run a trial together into one unified solution suite with a single sign on. Featuring solutions from site selection and activation, to payments, training and eConsent, all tied together with the DrugDev Golden Number DrugDev Spark is primed to transform the way global clinical trials are run. Join us for lunch and an exclusive preview at SCOPE!
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com