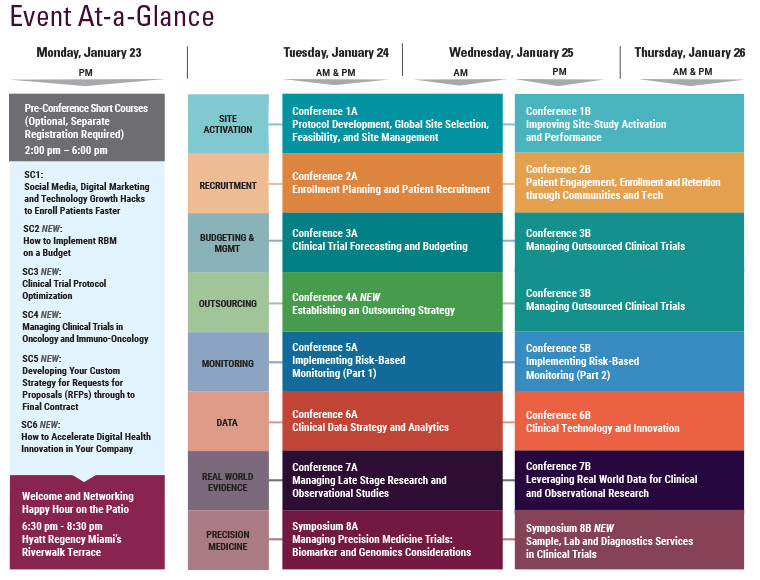
Cambridge Healthtech Institute’s 3rd Annual
Implementing Risk-Based Monitoring – Part 1:
Integrating Quality into Clinical Trials
January 24-25, 2017 | Hyatt Regency Miami | Miami, FL
Quality and risk management of clinical trials begins with the design and planning of clinical trials at the protocol stage. Cambridge Healthtech Institute’s Third Annual "Implementing Risk-Based Monitoring – Part 1: Integrating Quality into Clinical Trials" conference provides guidance on how to holistically and proactively build quality standards into clinical trials starting at the protocol stage with emphasis on the latest quality standards and guidelines, including ICH-E6, thereby ensuring higher quality clinical trials and laying the foundation for successful risk-based monitoring.
Final Agenda
Monday, January 23
1:00 pm Short Course Registration
Recommended Short Course*
2:00 – 6:00 pm SC3: Views and Conversations on Risk-Based Monitoring - Detailed Agenda
* Separate registration required
2:00-6:45 pm Main Conference Registration
6:30 – 8:30 pm Welcome and Networking Happy Hour on the Patio hosted by:




Tuesday, January 24
7:15 am Registration and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Sheri Kuss, Associate Director, Clinical Process Development, Teva Pharmaceuticals
10:50 Overcoming Challenges During Quality Protocol Development
 Sheri Kuss, Associate Director, Trial Optimization and Standards, Teva Pharmaceuticals
Sheri Kuss, Associate Director, Trial Optimization and Standards, Teva Pharmaceuticals
Implementing Risk-Based Monitoring takes effort upstream to incorporate Quality by Design and Risk Management principles during the protocol development process. Bringing the right stakeholders together during protocol design to identify risks, thresholds and mitigation plans is paramount. This course will review overcoming protocol development challenges in an effort to promote quality, simplification, compliance with regulations, alignment with endpoints and realistic patient participation.
11:15 Risk Planning and Mitigation – Overcoming Traditional Mindsets
 Nithiyanandhan Ananthakrishnan, CEO, Algorics
Nithiyanandhan Ananthakrishnan, CEO, Algorics
Risk planning is the corner stone for the successful conduct of clinical trials. The author has collaborated with a CRO to apply risk planning and wider risk based monitoring (RBM) principles on a study. The case study focuses on the risk planning framework, systematic methodology adopted, and advantages gained from implementing new technology. Experiences drawn from cross-functional collaboration, development of a holistic Integrated quality and risk management plan (IQRMP) and creation of risk indicators will also be discussed. Benefits derived at various steps and the outcome will also be shared.
11:40 Moving Beyond Risk Assessment: A Comprehensive Risk Management and Protocol Development Process that Builds Quality into Clinical Trials
 Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
This session describes a framework for a structured, proactive risk management process and the success factors for implementing and managing it. It also touches upon evaluating protocol design quality. The structured process includes three stages of Risk Management: Risk Assessment, Risk Mitigation and Control, and Issue Management. Based on the TransCelerate RACT questionnaire, the framework was collaboratively developed with input from 15 clinical research industry-related organizations (drug development and CROs).
 12:05 pm Impact to Quality from implementation of Risk Based Monitoring and Analytics
12:05 pm Impact to Quality from implementation of Risk Based Monitoring and Analytics
 Rajneesh Patil, Senior Director, Risk-Based Monitoring & Analytics, QuintilesIMS
Rajneesh Patil, Senior Director, Risk-Based Monitoring & Analytics, QuintilesIMS
More clients are moving study execution to a risk-based monitoring approach. This presentation highlights how you can: 1) Optimize site monitoring process flow and risk management 2) Streamline & automate processes that impact data flow and data quality positively 3) Improve oversight and control through advanced analytics and actionable insights.
12:40 Luncheon Presentation (Sponsorship Opportunity Available) or Lunch on Your Own
1:20 Coffee and Dessert in the Exhibit Hall
2:00 Chairperson’s Remarks
Sheri Kuss, Associate Director, Clinical Process Development, Teva Pharmaceuticals
2:05 Adherence to ICH E6 GCP Guidelines: An Exercise in Mapping Standards, Controls and Metrics
 Jonathan Rowe, Ph.D., Executive Director & Head, Quality Performance and Risk Management, Pfizer
Jonathan Rowe, Ph.D., Executive Director & Head, Quality Performance and Risk Management, Pfizer
The ICH E6 addendum is a move to modernize the GCP guidelines. Organizations involved in clinical research need to evaluate their own quality standards, processes and procedures in order to ensure that existing and new GCP requirements can be delivered. An overview and example of approaches used to identify strengths and gaps in GCP coverage will be provided that incorporates SOPs, controls and metrics. The approach is easily transferred to any size organization.
2:30 CO-PRESENTATION: Sponsor and Vendor Partnering to Implement a ICH E6 Compliant Risk Management Model
 Brian Nugent, Director, Clinical Operations & Process, Gilead Sciences
Brian Nugent, Director, Clinical Operations & Process, Gilead Sciences
 Angie Maurer, RN, BSN, MBA, Clinical Operations Consultant, PALM/Clinical Operations, Gilead Sciences
Angie Maurer, RN, BSN, MBA, Clinical Operations Consultant, PALM/Clinical Operations, Gilead Sciences
Partnering with CROs and Vendors on risk management in clinical trials brings with it the blending of diverse approaches to applying the principles now found in ICH E6. There are a number of pain points that may need to be addressed including: Data transfers, transparency, sharing of EDC data, customization of reports, and oversight. This case study presentation will blend the viewpoints of both sponsor and vendor and highlight the experiences of both in addressing this now common business challenge.
 3:20 Risk-Based Monitoring: Chasing a Moving Target
3:20 Risk-Based Monitoring: Chasing a Moving Target
Debra Jendrasek, Vice President, Strategic Development Partner, SA & SD, Strategic Development, Chiltern
Risk-based monitoring (RBM) has been a buzzword in the clinical research community for quite some time. No matter the term, for many, implementing RBM strategies means big change. As RBM grows in acceptance and implementation, learn about new strategies and processes and how to cull strong relationships with worldwide advisory bodies so your risk management becomes more efficient and less costly.
3:45 End of Session, Beginning of Interactive Breakout Discussion Groups
3:55 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and problem solving, and, most importantly, participate in active idea sharing. Click here for details.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, January 25
7:15 am Registration
7:30 Breakfast Presentation: eConsent: Put "Informed" Back in Informed Patient Consent
 Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Eric Delente, President, Patient Solutions, DrugDev; Co-founder of SecureConsent (Part of DrugDev)
Electronic informed consent makes the consenting process more efficient and effective for staff, sponsors, monitors, and most importantly patients by presenting the information in formats and language in which patients are comfortable. Join us for breakfast to learn the best practices, latest technological advances, and proven benefits of deploying an eConsent solution - including the impact it has on improving patient satisfaction and retention and help us put "informed" back in patient consent process.
8:25 Chairperson’s Remarks
Rajneesh Patil, Senior Director, Risk-Based Monitoring & Analytics, QuintilesIMS
8:30 Recap of Day 1 of Implementing Risk-Based Monitoring
Rajneesh Patil, Senior Director, Risk-Based Monitoring & Analytics, QuintilesIMS
Breakout Discussion Report Outs
Angie Maurer, RN, BSN, MBA, Clinical Operations Consultant, PALM/Clinical Operations, Gilead Sciences
8:55 An Automated Approach to Clinical Quality Management
 Ken Wu, Clinical Operations Consultant, Kenneth Wu and Associates, LLC
Ken Wu, Clinical Operations Consultant, Kenneth Wu and Associates, LLC
Organizations are developing their Clinical Quality Management System (CQMS) based on the TransCelerate CQMS Conceptual Framework. Some organizations are leveraging software as a service (SaaS) solutions to streamline and automate elements of the framework. This session will show how such a solution maps back to key elements in the framework and how an organization can move toward a proactive or preventative approach to Clinical Quality Management.
9:20 Supporting Global Clinical Operations and Integrating Quality and Audititing into Management Systems
 Joanne Spallone, Global Development Quality Audit Head, Franchise Operations and Strategy, Novartis
Joanne Spallone, Global Development Quality Audit Head, Franchise Operations and Strategy, Novartis
The logic and benefits of Quality by Design and proactive analysis and mitigation of risks to GCP quality are broadly recognized. So, what are your actual steps in accomplishing this in a large company with multiple projects and global teams? How do you gather the key stakeholders and organize a Quality strategy to support global clinical operations teams and how do you measure success? This presentation will share lessons learned as we implemented quality and auditing into our management systems.
.jpg) 9:45 Risk, Quality and the Real World – Capturing Data Directly and Engaging Patients
9:45 Risk, Quality and the Real World – Capturing Data Directly and Engaging Patients
 Jonathan Andrus, MS, CQA, CCDM, COO, Clinical Ink
Jonathan Andrus, MS, CQA, CCDM, COO, Clinical Ink
Industry often speaks on risk-based monitoring and changing the clinical trial game. Studies still embrace a paper to EDC approach requiring source document verification. This presentation will focus on tools that collaborate with patients and allow them to stay continually engaged in the study providing a risk-based approach to help improve the site workflow and reduce the administrative burdens that don’t yield better clinical results.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Rajneesh Patil, Senior Director, Risk-Based Monitoring & Analytics, QuintilesIMS
11:15 A Risk-Based Monitoring (RBM) Implementation: A Roadmap and Lessons Learned
 Justin Stark, Director/Head, Risk Based Monitoring, UCB Biosciences, Inc.
Justin Stark, Director/Head, Risk Based Monitoring, UCB Biosciences, Inc.
Practices associated with RBM are becoming established within clinical development and are anticipated to become expected by regulators with the anticipated revision to the ICH E6 GCP guidelines. This presentation will discuss the implementation of a risk-based approach to ensure protection of patient safety and the reliability of clinical trial results within a mid-size pharma company. The discussion will focus on embedding QbD and pro-active identification and management of risk coupled with fit-for-purpose data surveillance and oversight in collaboration with CRO partners and share an implementation roadmap and lessons learned.
 11:40 RBM Demystified: What We've Learned from the Practical Implementation of RBM and What the Regulators Want
11:40 RBM Demystified: What We've Learned from the Practical Implementation of RBM and What the Regulators Want
 Francois Torche, CEO, CluePoints
Francois Torche, CEO, CluePoints
Join CluePoints CEO, Francois Torche for an insightful session that will showcase a tried and tested approach to RBM for Sponsors, Sites and CROs. This presentation will cover: 1) Best practices for the RBM study planning process 2) Key pitfalls to avoid 3) How to differentiate between Risk Assessment, KRIs and Data Quality Oversight 4) How other Sponsors/CROs are leveraging a Risk-Based approach to monitoring and satisfying regulatory requirements
12:05 pm Bridging Luncheon Presentation: Risk-Based Monitoring – Making it Work
 Keith Howells, Senior Vice President, Development, OmniComm Systems, Inc.
Keith Howells, Senior Vice President, Development, OmniComm Systems, Inc.
A major benefit of risk-based monitoring is to perform less than 100% Source Document Verification, freeing monitoring staff for more valuable work and reducing the burden on the sites. We will share an example of the new workflow during a monitoring visit, analyze trials where RBM have been implemented and identify specific approaches that have been employed.
12:50 Coffee and Dessert in the Exhibit Hall
1:30 Close of Conference
SCOPE 2016 Wrap-Up
The 7th Annual SCOPE Summit, held February 23-25, 2016 in Miami, Florida, had record attendance with more than 1,150 industry leaders joining 3 days of in-depth discussions covering important issues in clinical trial planning and management. As SCOPE grew in attendance by 30% over last year, the 2016 program offered 12 distinct conference tracks, 2 symposia, 3 short courses, and 4 plenary keynote sessions, focused on advances and innovative solutions in all aspects of clinical trial management and operations, including Data Integration, Feasibility, Site Selection and Management, Patient Engagement, Recruitment and Retention, Mobile Tech, Project Management, Outsourcing, Forecasting, Budgeting and Contracting, Quality (QbD) in Trial Conduct, Risk-Based Monitoring, Post-Marketing Studies, Observational Research, Statistics and Biomarker-Driven Trials.
For Further Information
For questions or suggestions about the meeting, please contact:
Micah Lieberman
Executive Director, Conferences
Cambridge Healthtech Institute (CHI)
T: (+1) 541.482.4709
E: mlieberman@healthtech.com
For partnering and sponsorship information, please contact:
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, please contact:
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com