Cambridge Healthtech Institute’s 4th Annual
Implementing Risk-Based Monitoring – Part 1:
Integrating Quality into Clinical Trials
February 13-14, 2018 | Hyatt Regency Orlando | Orlando, FL
Poor quality and risk management of clinical trials significantly impacts the success, timeliness and cost-effectiveness of clinical trials. Given the recent adoption of ICH E6 R2 changes, now is a critical time for the pharma industry to reflect on their
processes and ensure quality across their clinical research activities, in particular, data management, and sponsor and investigator responsibilities. Cambridge Healthtech Institute’s 4th Annual Implementing Risk-Based Monitoring – Part 1: Integrating Quality into Clinical Trials conference
provides guidance on how to holistically and proactively build quality standards into clinical trials with emphasis on the latest quality standards and guidelines, including the recent ICH-E6 R2 addendum changes, thereby ensuring higher quality clinical
trials and laying the foundation for successful risk-based monitoring.
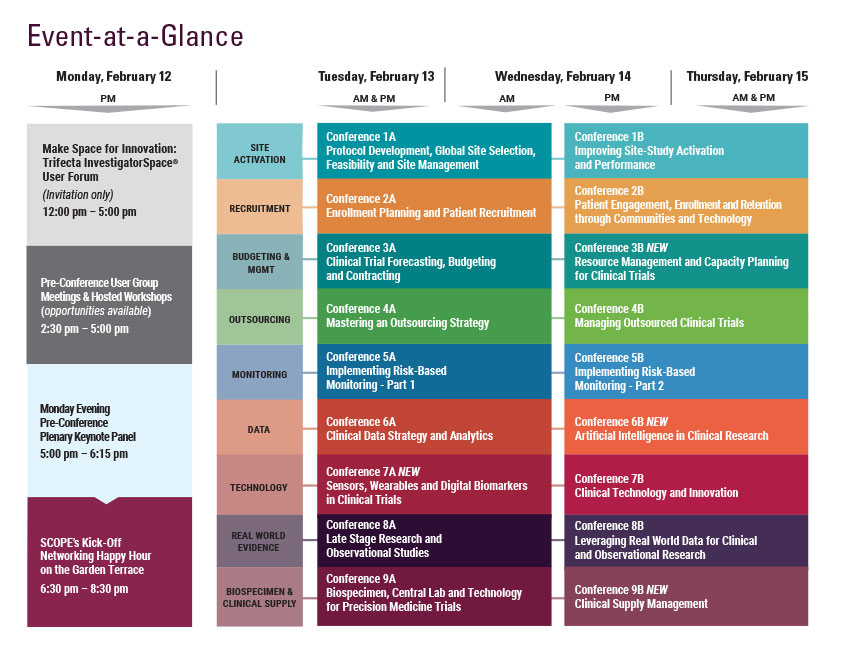
Monday, February 12
9:00 am - 7:30 pm Registration Open
5:00 - 6:15 pm Pre-Conference Plenary Keynote Panel
6:30 – 8:30 pm SCOPE’s Kick-Off Networking Happy Hour on the Garden Terrace Hosted by CHI, DrugDev, Exostar, & Praxis
8:30 Close of Day
Tuesday, February 13
7:15 am Registration Open and Morning Coffee
8:20 Opening Plenary Keynotes
9:45 Grand Opening Coffee Break in the Exhibit Hall
10:45 Chairperson’s Remarks
Janis Little, Vice President, Global R&D Quality, Allergan
10:50 PANEL DISCUSSION: The Total Cost of Quality: Finding the Balance Between Investing in Quality and the Cost of Fixing Quality Problems
Moderator:  Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
Panelists:
 Armelde Pitre, MBA, Head, Quality Performance Analytics, Clinical Development Quality, Global Product Development,
Pfizer, Inc.
Armelde Pitre, MBA, Head, Quality Performance Analytics, Clinical Development Quality, Global Product Development,
Pfizer, Inc.
 Janis Little, Vice President, Global R&D Quality, Allergan
Janis Little, Vice President, Global R&D Quality, Allergan
 Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, Clinical Development Services, PPD
Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, Clinical Development Services, PPD
 Yusuf Ghadiali, Senior Director, Performance Operational Capability, Global Clinical Operations, Biogen
Yusuf Ghadiali, Senior Director, Performance Operational Capability, Global Clinical Operations, Biogen
Few organizations have a complete understanding of the financial impact of poor clinical trial quality. With better understanding of poor quality on an organization’s bottom line, it can improve buy-in for proactively investing in quality
to avoid costly quality problems. In this panel, representatives from across pharma will come together to discuss the sources of poor clinical trial quality (ex. protocol amendments, low/non-enrolling sites, subjects dropping out, data quality
issues, etc.), the challenges of estimating poor quality costs, current quality cost estimate techniques including the Cost of Poor Quality Estimator Tool developed by the MCC Study Quality Trailblazer Group, and how improved access to data
about the cost of poor quality is impacting risk management and quality investment decisions.
11:40 Quality by Collaboration: Practical Applications
Jolie Weintraub, Executive Director, TA Head Oncology, MRL Quality Assurance, Merck
This presentation will discuss how quality is implemented within Merck, including the importance of collaborating with various quality functions at all levels of the organization. It will provide a holistic approach to supporting and achieving
quality proactively throughout the clinical trial process. In addition, case studies will be shared on how this principle has been put into practice and steps to take to foster the collaborations.
 12:05 pm The
Value of Centralized Monitoring on Monitoring Visits—Increasing Quality and Decreasing Costs
12:05 pm The
Value of Centralized Monitoring on Monitoring Visits—Increasing Quality and Decreasing Costs
 Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, Clinical Development Services, PPD
Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, Clinical Development Services, PPD
As regulatory authorities embrace change and the industry focuses on reducing costs, increasing data integrity and patient safety, the importance of centralized monitoring increases. PPD combines centralized monitoring with site health assessments
to enhance site performance. Learn how our CRAs are moving from finding to fixing issues and becoming experts in process improvement to help sites become more engaged and efficient—ultimately reducing risk, increasing safety, ensuring
quality and reducing the costs of drug development.
12:30 Session Break
 12:40 Luncheon
Presentation: The Role of Innovation and New Age Analytics in RBM Evolution
12:40 Luncheon
Presentation: The Role of Innovation and New Age Analytics in RBM Evolution
 Rajneesh Patil, Senior Director, Clinical Operations Process Design and Analytics, IQVIA
Rajneesh Patil, Senior Director, Clinical Operations Process Design and Analytics, IQVIA
What problems are we looking to solve with innovation in RBM models. How do the new age analytics and advent of machine learning augment risk-based strategies. What results are we experiencing through implementation of statistical monitoring
and analytics.
1:20 Coffee and Dessert Break in the Exhibit Hall
2:00 Chairperson’s Remarks
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
2:05 TransCelerate’s Clinical QMS
 Janis Little, Vice President, Global R&D Quality, Allergan
Janis Little, Vice President, Global R&D Quality, Allergan
The presentation will cover what a Clinical QMS is, and what the benefits are to sponsors in having a Clinical QMS defined and in place in alignment with ICH E6 (rev 2) requirements in Section 5. Audience will recognize and understand the
flexibility of the TransCelerate Conceptual Framework for a Clinical QMS that will help sponsors design a customized CQMS for their specific needs. The presentation will dispel myths that exist on what a Clinical QMS “is” and
what it is not, and it will cover what TransCelerate QMS publications and tools are currently available to industry. The presentation will also take a deep dive into a newly available tool for sponsors to assess a clinical quality management
system (if they have implemented in alignment with the TransCelerate CQMS Conceptual Framework).
2:55 Envisioning a Quality Management System to Address the ICH E6 R2 Changes
 Andy Lawton, Director and Consultant, Risk Based Approach Ltd.
Andy Lawton, Director and Consultant, Risk Based Approach Ltd.
The changes in ICH E6 R2 impact the fundamental basis of how quality should be addressed by a sponsor. This presentation will take a holistic look at the Regulatory background, and the tools that can be used to address the critical issues
of Quality Tolerance Limits, Quality by Design, and Continuous Quality Improvement.
3:20 Risk-Based Monitoring Strategies for Improved Clinical Trial Performance
 Henry Galio, Senior Director, Vault CTMS, Veeva Systems
Henry Galio, Senior Director, Vault CTMS, Veeva Systems
A strategic, risk-based approach to clinical trial management can aid sponsors and CROs in early detection and mitigation of risks that could affect the quality and safety of a study and its subjects. However, risk mitigation strategies have
little value unless they are executed, monitored, and analyzed continuously throughout the trial’s lifecycle. This session explores how cloud-enabled solutions can be leveraged to gain real-time insights and actionable analytics
to improve clinical trial safety and performance.
3:50 Find Your Table and Meet Your Moderator
4:00 Interactive Breakout Discussion Groups
Concurrent breakout discussion groups are interactive, guided discussions hosted by a facilitator or set of co-facilitators to discuss some of the key issues presented earlier in the day’s sessions. Delegates will join a table of interest
and become an active part of the discussion at hand. To get the most out of this interactive session and format please come prepared to share examples from your work, vet some ideas with your peers, be a part of group interrogation and
problem solving, and, most importantly, participate in active idea sharing.
5:00 Welcome Reception in the Exhibit Hall
6:30 Close of Day
Wednesday, February 14
7:15 am Registration Open
7:45 Breakfast Presentation: The Next Generation of Site Payments: Technology Do's and Don'ts
Stuart Thiede, President, Payments, DrugDev (An IQVIA Company)
Ineffective site payment processes can irreparably damage the site/sponsor relationship, resulting in the dreaded “one and done” mentality for many sites. However, leveraging a purpose-built technology solution can remove such
concerns by ensuring reliable cycle times, efficient processing, real-time reporting, and transparency, all of which drive site satisfaction and simplify the lives of study teams. This session will highlight best practice technology
and process models to ensure you achieve your site payment objectives.
8:15 Session Break
8:25 Chairperson’s Remarks
Nithiya Ananthakrishnan, Senior Vice President, Data Science, OmniComm Systems
 8:30 CO-PRESENTATION:
How an Ongoing Joint Risk-Based Monitoring Deployment Aligns with ICH E6 (R2)
8:30 CO-PRESENTATION:
How an Ongoing Joint Risk-Based Monitoring Deployment Aligns with ICH E6 (R2)
 Mike Walega, Executive Director, Monitoring & Data Flow, Covance
Mike Walega, Executive Director, Monitoring & Data Flow, Covance
 Jude Burger, MS, Manager, Eli Lilly
Jude Burger, MS, Manager, Eli Lilly
We will provide an overview of how an ongoing RBM deployment aligns with the new ICH GCP E6 (R2) guidelines. We will review the history of the deployment and the relevant aspects of E6 (R2). We will then focus on describing how this approach
aligns with E6 (R2), where gaps exist, and how we plan to fill the gaps. Through examples and recommendations, we will provide attendees with insights to their own evolving RBM deployments.
9:20 How ICH E6 Updates Redefine How We Manage and Measure Quality
 Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
Linda Sullivan, Co-Founder & President, Metrics Champion Consortium LLC
9:45 Risk-Based Monitoring: Looking Beyond Risk
 George H. Johnson IV, SCPM, Product Manager & Senior Analyst, Clinical Analytics, PerkinElmer
George H. Johnson IV, SCPM, Product Manager & Senior Analyst, Clinical Analytics, PerkinElmer
There is mass concentration and growing need to adopt Risk-Based Monitoring given the obvious benefits to clinical trials. However, there is unspoken value beyond just KRIs and process changes. Join us as we discuss real-world cases of
tangible benefits when looking beyond risk, including: RBM implementation best practices; what it means to look beyond the risk to implement QbD; quality improvements derived by rich RBM insights; driving CRO-Sponsor-Site relationships
to the next level.
10:10 Coffee Break in the Exhibit Hall
11:10 Chairperson’s Remarks
Nithiya Ananthakrishnan, Senior Vice President, Data Science, OmniComm Systems
11:15 PANEL DISCUSSION: ICH E6 and How the Industry Is Tackling Things Head On
 Moderator: Andy Lawton, Director and Consultant, Risk Based Approach Ltd.
Moderator: Andy Lawton, Director and Consultant, Risk Based Approach Ltd.
Panelists:
 Jonathan Rowe, Ph.D., Executive Director & Head, Quality Performance and Risk Management,
Pfizer
Jonathan Rowe, Ph.D., Executive Director & Head, Quality Performance and Risk Management,
Pfizer
Nithiya Ananthakrishnan, Senior Vice President, Data Science, OmniComm Systems
 Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, Clinical Development
Services, PPD
Nicole Stansbury, Executive Director, Adaptive and Intelligent Monitoring, Clinical Development
Services, PPD
With the passage of ICH E6 (R2) addendum, the pharma industry is taking a closer look at how they approach clinical trial quality and oversight with their partners. This panel will cover how individual organizations are approaching
ICH E6 R2 addendum changes, the struggles and challenges they faced or continue to face, and how they are working with other stakeholders (their CRO partners, stakeholders across different departments, etc.).
12:05 pm Session Break
12:10 Bridging Luncheon Presentation: Extracting Data from the EHR Dramatically Reduces the Need for Manual Monitoring - New Standards Make This Possible
 Kim Rejndrup, Senior Vice President, Product Development, OmniComm
Kim Rejndrup, Senior Vice President, Product Development, OmniComm
The typical workflow at an investigate site is for the patient data to be entered into the Electronic Health Record, then for the study coordinator to re-type that data into the EDC system. A big component of the subsequent Source
Data Verification is simply checking that the retyping was done accurately. If data could instead flow automatically from the EHR system, with the coordinator just verifying the data in transit, then both the data entry and
the subsequent SDV can be automated. New standards and technologies being introduced under the “SMART on FHIR” umbrella now make this possible. This presentation will describe the state of the art in this exciting
new field.
12:50 Coffee and Dessert Break in the Exhibit Hall
1:30 Close of Conference
 Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
Group Discounts Are Available! Special rates are available for multiple
attendees from the same organization. For more information on group discounts, contact Melissa Dolen at 781-972-5418 or mdolen@healthtech.com.
For questions or suggestions about the meeting, contact:
Lee Yuan
Conference Director
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5404
E: lyuan@healthtech.com
For partnering and sponsorship information, contact:
 Ilana Quigley
Ilana Quigley
Senior Manager, Business Development
Cambridge Healthtech Institute (CHI)
T:
(+1) 781.972.5457
E: iquigley@healthtech.com
For media and association partnerships, contact:
 Rich Handy
Rich Handy
Senior Director, Marketing
Cambridge Healthtech Institute (CHI)
T: (+1) 781.972.5456
E: rhandy@healthtech.com